YOUNG AGAIN
Light Based Over The Counter Wrinkle Reduction
ESPANSIONE MARKETING SPA
The following data is part of a premarket notification filed by Espansione Marketing Spa with the FDA for Young Again.
Pre-market Notification Details
| Device ID | K124064 |
| 510k Number | K124064 |
| Device Name: | YOUNG AGAIN |
| Classification | Light Based Over The Counter Wrinkle Reduction |
| Applicant | ESPANSIONE MARKETING SPA VIA ALTOBELLI BONETTI 3A Imola(bo), IT 40026 |
| Contact | Guido Bonapace |
| Correspondent | Guido Bonapace ESPANSIONE MARKETING SPA VIA ALTOBELLI BONETTI 3A Imola(bo), IT 40026 |
| Product Code | OHS |
| CFR Regulation Number | 878.4810 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2012-12-31 |
| Decision Date | 2013-12-19 |
| Summary: | summary |
Trademark Results [YOUNG AGAIN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
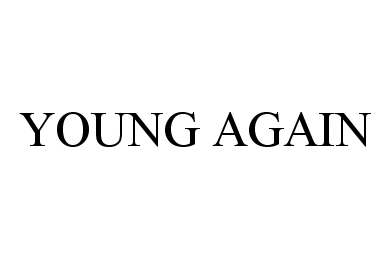 YOUNG AGAIN 78444118 2989632 Dead/Cancelled |
Young Again Nutrition, LLC 2004-06-30 |
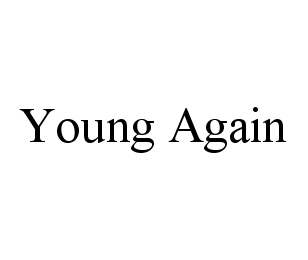 YOUNG AGAIN 78421085 not registered Dead/Abandoned |
Ouvrier, John Paul 2004-05-18 |
 YOUNG AGAIN 76563456 not registered Dead/Abandoned |
STC Services, Inc. 2003-12-05 |
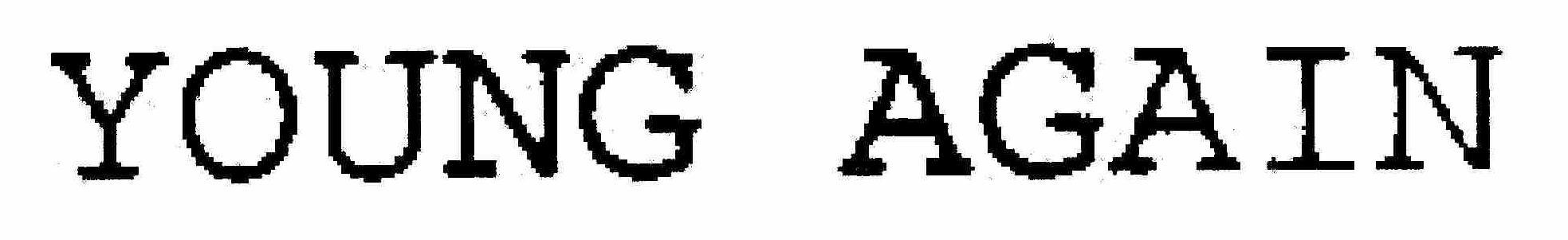 YOUNG AGAIN 76073269 3374637 Live/Registered |
Young Again Products, Inc. 2000-06-19 |
 YOUNG AGAIN 75975168 not registered Dead/Abandoned |
TONY LITTLE ENTERPRISES, INC. 1995-08-28 |
 YOUNG AGAIN 75604940 not registered Dead/Abandoned |
LITTLE, ANTHONY 1998-12-14 |
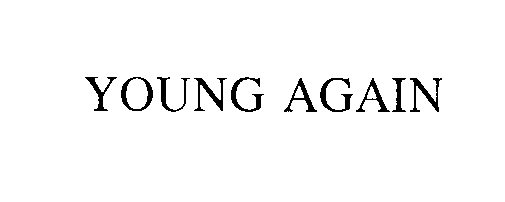 YOUNG AGAIN 75556132 2478357 Dead/Cancelled |
YOUNG AGAIN NUTRITION, LLC 1998-09-21 |
 YOUNG AGAIN 74721933 not registered Dead/Abandoned |
TONY LITTLE ENTERPRISES, INC. 1995-08-28 |
 YOUNG AGAIN 74677098 not registered Dead/Abandoned |
ALLEGHANY PHARMACAL CORPORATION 1995-05-19 |
 YOUNG AGAIN 72215625 0812217 Dead/Expired |
LOV-E BRASSIERE COMPANY 1965-04-02 |
 YOUNG AGAIN 72142893 0744200 Dead/Expired |
DE MERT & DOUGHERTY, INC. 1962-04-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.