D-RAD SMART PACK
Plate, Fixation, Bone
SMITH & NEPHEW, INC.
The following data is part of a premarket notification filed by Smith & Nephew, Inc. with the FDA for D-rad Smart Pack.
Pre-market Notification Details
| Device ID | K132296 |
| 510k Number | K132296 |
| Device Name: | D-RAD SMART PACK |
| Classification | Plate, Fixation, Bone |
| Applicant | SMITH & NEPHEW, INC. 1450 BROOKS RD. Memphis, TN 38116 |
| Contact | Martin Ostmann |
| Correspondent | Martin Ostmann SMITH & NEPHEW, INC. 1450 BROOKS RD. Memphis, TN 38116 |
| Product Code | HRS |
| CFR Regulation Number | 888.3030 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2013-07-23 |
| Decision Date | 2014-01-07 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 00885556582985 | K132296 | 000 |
| 00885556394649 | K132296 | 000 |
| 00885556394441 | K132296 | 000 |
| 00885556394397 | K132296 | 000 |
| 00885556394342 | K132296 | 000 |
| 00885556394298 | K132296 | 000 |
| 00885556394243 | K132296 | 000 |
| 00885556394199 | K132296 | 000 |
| 00885556394144 | K132296 | 000 |
| 00885556364598 | K132296 | 000 |
| 00885556364543 | K132296 | 000 |
| 00885556364499 | K132296 | 000 |
| 00885556364444 | K132296 | 000 |
| 00885556364390 | K132296 | 000 |
| 00885556394748 | K132296 | 000 |
| 00885556394847 | K132296 | 000 |
| 00885556396445 | K132296 | 000 |
| 00885556396346 | K132296 | 000 |
| 00885556396247 | K132296 | 000 |
| 00885556396148 | K132296 | 000 |
| 00885556396049 | K132296 | 000 |
| 00885556395943 | K132296 | 000 |
| 00885556395844 | K132296 | 000 |
| 00885556395745 | K132296 | 000 |
| 00885556395349 | K132296 | 000 |
| 00885556395240 | K132296 | 000 |
| 00885556395141 | K132296 | 000 |
| 00885556395042 | K132296 | 000 |
| 00885556394946 | K132296 | 000 |
| 00885556364345 | K132296 | 000 |
Trademark Results [D-RAD SMART PACK]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
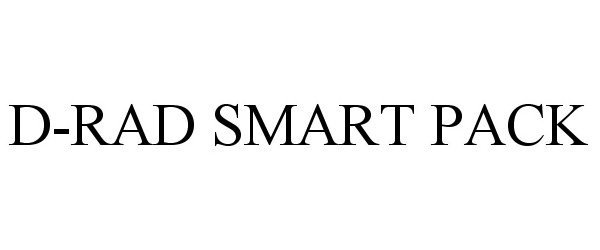 D-RAD SMART PACK 85867992 4739568 Live/Registered |
Smith & Nephew, Inc. 2013-03-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.