SCENIUM
System, Image Processing, Radiological
SIEMENS MEDICAL SOLUTIONS USA, INC.
The following data is part of a premarket notification filed by Siemens Medical Solutions Usa, Inc. with the FDA for Scenium.
Pre-market Notification Details
| Device ID | K133654 |
| 510k Number | K133654 |
| Device Name: | SCENIUM |
| Classification | System, Image Processing, Radiological |
| Applicant | SIEMENS MEDICAL SOLUTIONS USA, INC. 2501 NORTH BARRINGTON RD. Hoffman Estates, IL 60192 |
| Contact | Maria Ebio |
| Correspondent | Maria Ebio SIEMENS MEDICAL SOLUTIONS USA, INC. 2501 NORTH BARRINGTON RD. Hoffman Estates, IL 60192 |
| Product Code | LLZ |
| CFR Regulation Number | 892.2050 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2013-11-27 |
| Decision Date | 2014-02-28 |
| Summary: | summary |
Trademark Results [SCENIUM]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SCENIUM 88364502 not registered Live/Pending |
TECHNICOLOR 2019-03-31 |
 SCENIUM 87816470 5584897 Live/Registered |
Siemens Medical Solutions USA, Inc. 2018-03-01 |
 SCENIUM 86482753 5745716 Live/Registered |
RCA Trademark Management 2014-12-17 |
 SCENIUM 85475410 4175121 Dead/Cancelled |
Montblanc-Simplo GmbH 2011-11-17 |
 SCENIUM 85232506 not registered Dead/Abandoned |
TECHNICOLOR 2011-02-02 |
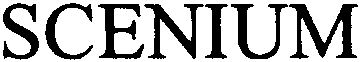 SCENIUM 79000803 2951880 Dead/Cancelled |
Montblanc-Simplo GmbH 2004-02-04 |
 SCENIUM 78917298 3275008 Dead/Cancelled |
Siemens Molecular Imaging Limited 2006-06-26 |
 SCENIUM 78657228 not registered Dead/Abandoned |
CTI Mirada Solutions Limited 2005-06-23 |
 SCENIUM 76061072 2764716 Dead/Cancelled |
THOMSON INC. 2000-06-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.