LLD EZ
Stylet, Catheter
SPECTRANETICS, INC.
The following data is part of a premarket notification filed by Spectranetics, Inc. with the FDA for Lld Ez.
Pre-market Notification Details
| Device ID | K142116 |
| 510k Number | K142116 |
| Device Name: | LLD EZ |
| Classification | Stylet, Catheter |
| Applicant | SPECTRANETICS, INC. 9965 Federal Drive Colorado Springs, CO 80921 |
| Contact | Pharoah Garma |
| Correspondent | Pharoah Garma SPECTRANETICS, INC. 9965 Federal Drive Colorado Springs, CO 80921 |
| Product Code | DRB |
| CFR Regulation Number | 870.1380 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Special |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2014-08-04 |
| Decision Date | 2014-08-12 |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 20813132023076 | K142116 | 000 |
| M204518067AB0 | K142116 | 000 |
| M204518062AA0 | K142116 | 000 |
| 00813132020835 | K142116 | 000 |
| M2045180670 | K142116 | 000 |
| M2045180621 | K142116 | 000 |
Trademark Results [LLD EZ]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
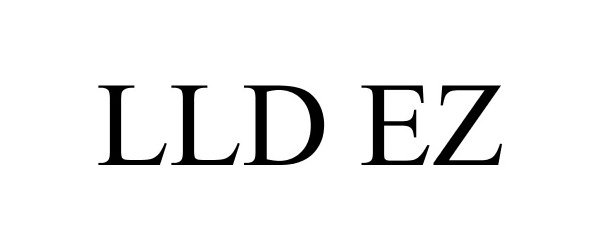 LLD EZ 77309793 3562200 Live/Registered |
The Spectranetics Corporation 2007-10-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.