OrthoPulse
Orthodontic Led Accessory
BIOLUX RESEARCH LTD.
The following data is part of a premarket notification filed by Biolux Research Ltd. with the FDA for Orthopulse.
Pre-market Notification Details
| Device ID | K143120 |
| 510k Number | K143120 |
| Device Name: | OrthoPulse |
| Classification | Orthodontic Led Accessory |
| Applicant | BIOLUX RESEARCH LTD. 825 POWELL STREET, SUITE 220 Vancouver, CA V6a 1h7 |
| Contact | Kevin Strange |
| Correspondent | Janice Hogan HOGAN LOVELLS US LLP 1835 MARKET ST 29TH FL Philadelphia, PA 19103 |
| Product Code | PLH |
| CFR Regulation Number | 872.5470 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2014-10-30 |
| Decision Date | 2015-07-24 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 00815950000013 | K143120 | 000 |
| 09120118090029 | K143120 | 000 |
| 09120118090067 | K143120 | 000 |
Trademark Results [OrthoPulse]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ORTHOPULSE 86448519 5078503 Live/Registered |
BIOLUX RESEARCH HOLDINGS, INC. 2014-11-07 |
 ORTHOPULSE 86173036 4960675 Live/Registered |
CuraMedix, LLC 2014-01-23 |
 ORTHOPULSE 79324604 not registered Live/Pending |
LLLT Technologies SA 2021-08-23 |
 ORTHOPULSE 76316740 2804038 Dead/Cancelled |
IMD-International Market Development BV 2001-09-25 |
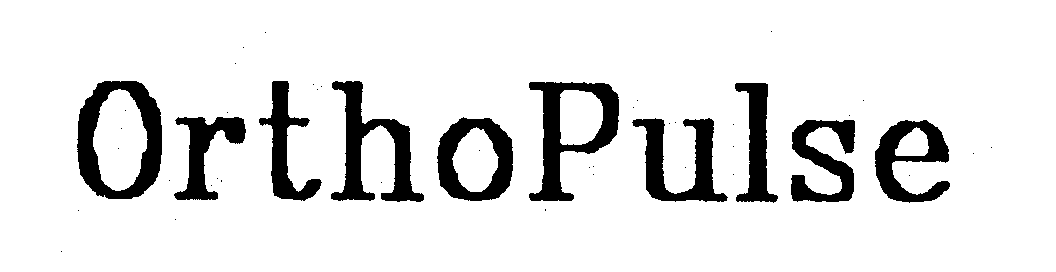 ORTHOPULSE 74198488 1753766 Dead/Cancelled |
IMD Innovative Medical Devices B.V. 1991-08-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.