Mini Me
Powered Light Based Non-laser Surgical Instrument With Thermal Effect
ILUMINAGE LTD.(FORMERLY SYNERON BEAUTY LTD.)
The following data is part of a premarket notification filed by Iluminage Ltd.(formerly Syneron Beauty Ltd.) with the FDA for Mini Me.
Pre-market Notification Details
| Device ID | K143339 |
| 510k Number | K143339 |
| Device Name: | Mini Me |
| Classification | Powered Light Based Non-laser Surgical Instrument With Thermal Effect |
| Applicant | ILUMINAGE LTD.(FORMERLY SYNERON BEAUTY LTD.) KOCHAV YOKNEAM BLDG. YOKNEAM INDUSTRIAL ZONE Yokneam Illit, IL 20692 |
| Contact | Bobae Kim |
| Correspondent | Janice M Hogan HOGAN LOVELLS US LLP 1835 MARKET ST., 29TH FL Philadelphia, PA 19103 |
| Product Code | ONF |
| CFR Regulation Number | 878.4810 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Special |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2014-11-20 |
| Decision Date | 2014-12-19 |
| Summary: | summary |
Trademark Results [Mini Me]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MINI ME 97308581 not registered Live/Pending |
L&S Prints Digital LTD 2022-03-11 |
 MINI ME 97043084 not registered Live/Pending |
Tan, David 2021-09-24 |
 MINI ME 90796450 not registered Live/Pending |
K2R2 LLC 2021-06-25 |
 MINI ME 87056494 5287344 Live/Registered |
Minime 2016-06-01 |
 MINI ME 86794833 5120753 Live/Registered |
Pecos Pit International Franchise, LLC 2015-10-21 |
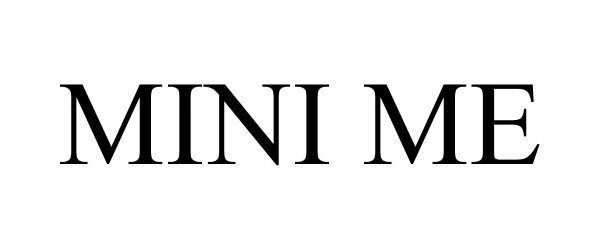 MINI ME 85792760 4435152 Live/Registered |
Societe des Produits Nestle S.A. 2012-12-03 |
 MINI ME 85579516 4485717 Live/Registered |
MICROELECTRONICS TECHNOLOGY INC. 2012-03-26 |
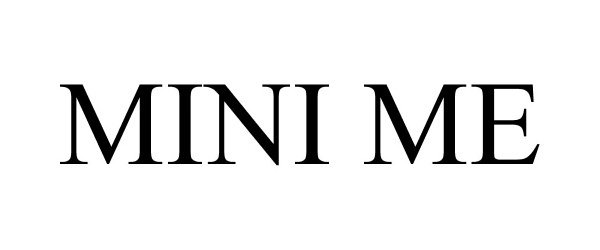 MINI ME 85227018 not registered Dead/Abandoned |
Town & Country Pillows, Inc. 2011-01-26 |
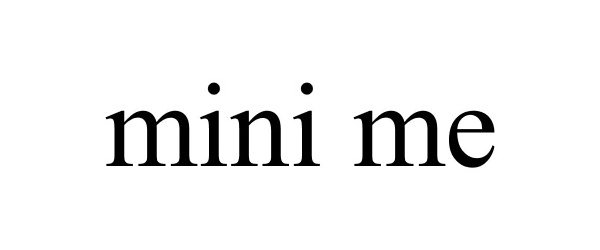 MINI ME 78818733 not registered Dead/Abandoned |
Earthquake Sound Corporation 2006-02-20 |
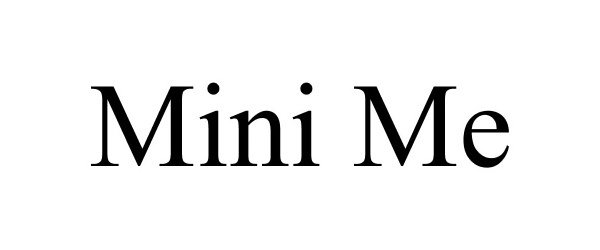 MINI ME 78697646 3120757 Live/Registered |
Ayco Farms, Inc. 2005-08-22 |
 MINI ME 78388616 not registered Dead/Abandoned |
NUTRAMARKS, INC. 2004-03-22 |
 MINI ME 78388616 not registered Dead/Abandoned |
Owens, Caroline 2004-03-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.