Rachel's Remedy
Pack, Heat, Moist
Rachel's Remedies, LLC
The following data is part of a premarket notification filed by Rachel's Remedies, Llc with the FDA for Rachel's Remedy.
Pre-market Notification Details
| Device ID | K150139 |
| 510k Number | K150139 |
| Device Name: | Rachel's Remedy |
| Classification | Pack, Heat, Moist |
| Applicant | Rachel's Remedies, LLC 542 Parkside Ave Buffalo, NY 14216 |
| Contact | Rachel E. Jackson |
| Correspondent | Rachel E. Jackson Rachel's Remedies, LLC 542 Parkside Ave Buffalo, NY 14216 |
| Product Code | IMA |
| CFR Regulation Number | 890.5730 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2015-01-22 |
| Decision Date | 2015-05-22 |
| Summary: | summary |
Trademark Results [Rachel's Remedy]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
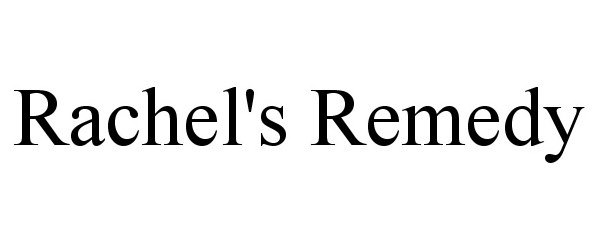 RACHEL'S REMEDY 87477469 5378586 Live/Registered |
Rachel's Remedies, LLC 2017-06-06 |
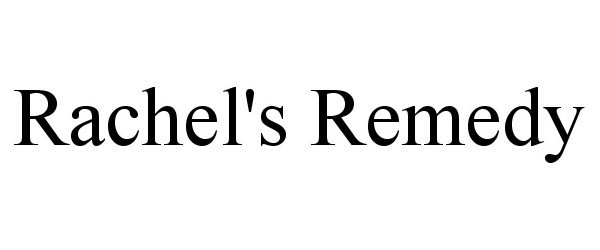 RACHEL'S REMEDY 87477446 5378584 Live/Registered |
Rachel's Remedies, LLC 2017-06-06 |
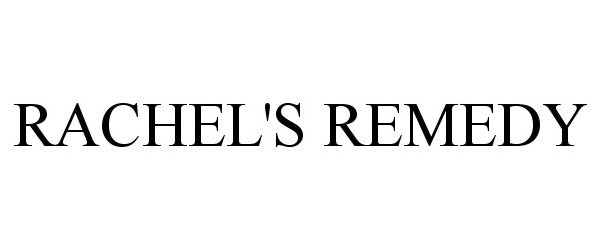 RACHEL'S REMEDY 86506497 4764734 Live/Registered |
Rachel's Remedies, LLC 2015-01-17 |
 RACHEL'S REMEDY 86506482 4785893 Live/Registered |
Rachel's Remedies, LLC 2015-01-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.