A-Oss
Bone Grafting Material, Animal Source
HIOSSEN INC.
The following data is part of a premarket notification filed by Hiossen Inc. with the FDA for A-oss.
Pre-market Notification Details
| Device ID | K151542 |
| 510k Number | K151542 |
| Device Name: | A-Oss |
| Classification | Bone Grafting Material, Animal Source |
| Applicant | HIOSSEN INC. 85 BEN FAIRLESS DR. Fairless Hills, PA 19030 |
| Contact | David Kim |
| Correspondent | David Kim HIOSSEN INC. 85 BEN FAIRLESS DR. Fairless Hills, PA 19030 |
| Product Code | NPM |
| CFR Regulation Number | 872.3930 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2015-06-08 |
| Decision Date | 2016-08-03 |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 00814913025063 | K151542 | 000 |
| 00814913024295 | K151542 | 000 |
| 00814913024288 | K151542 | 000 |
| 00814913024271 | K151542 | 000 |
| 00814913024264 | K151542 | 000 |
| 00814913024257 | K151542 | 000 |
| 00814913024240 | K151542 | 000 |
| 00814913024233 | K151542 | 000 |
| 00814913024226 | K151542 | 000 |
| 00814913024301 | K151542 | 000 |
| 00814913024974 | K151542 | 000 |
| 00814913025056 | K151542 | 000 |
| 00814913025049 | K151542 | 000 |
| 00814913025032 | K151542 | 000 |
| 00814913025025 | K151542 | 000 |
| 00814913025018 | K151542 | 000 |
| 00814913025001 | K151542 | 000 |
| 00814913024998 | K151542 | 000 |
| 00814913024981 | K151542 | 000 |
| 00814913024219 | K151542 | 000 |
Trademark Results [A-Oss]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
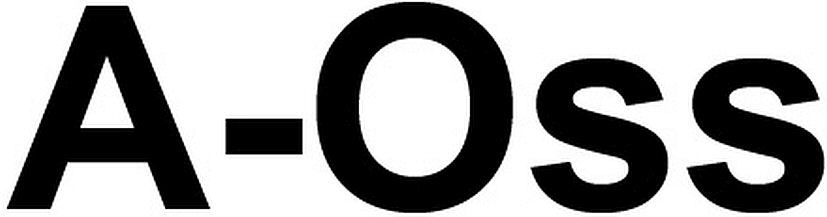 A-OSS 79186040 5047552 Live/Registered |
OSSTEMIMPLANT CO., LTD. 2016-02-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.