MultiFIX® P Knotless Fixation Device
Fastener, Fixation, Nondegradable, Soft Tissue
ArthroCare Corporation
The following data is part of a premarket notification filed by Arthrocare Corporation with the FDA for Multifix® P Knotless Fixation Device.
Pre-market Notification Details
| Device ID | K151897 |
| 510k Number | K151897 |
| Device Name: | MultiFIX® P Knotless Fixation Device |
| Classification | Fastener, Fixation, Nondegradable, Soft Tissue |
| Applicant | ArthroCare Corporation 15285 Alton Parkway, Suite 200 Irvine, CA 92618 |
| Contact | Laura Kasperowicz |
| Correspondent | Laura Kasperowicz ArthroCare Corporation 15285 Alton Parkway, Suite 200 Irvine, CA 92618 |
| Product Code | MBI |
| CFR Regulation Number | 888.3040 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Special |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2015-07-10 |
| Decision Date | 2015-08-06 |
| Summary: | summary |
Trademark Results [MultiFIX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MULTIFIX 85463854 4223235 Live/Registered |
ArthroCare Corporation 2011-11-03 |
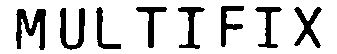 MULTIFIX 79091280 not registered Dead/Abandoned |
SRW-Amestra GmbH 2010-05-26 |
 MULTIFIX 77273109 3430393 Live/Registered |
Advanced BioTech LLC 2007-09-06 |
 MULTIFIX 77078738 not registered Dead/Abandoned |
Carl Fuhr GmbH & Co. KG 2007-01-09 |
 MULTIFIX 75861295 not registered Dead/Abandoned |
Piraiegar, Mohammad 1999-12-01 |
 MULTIFIX 75234766 not registered Dead/Abandoned |
MERIDIAN MEDICAL 1997-02-03 |
 MULTIFIX 74421299 1899684 Live/Registered |
FUGRO N.V. 1993-08-06 |
 MULTIFIX 74366921 1884990 Dead/Cancelled |
Plasplugs Limited 1993-03-11 |
 MULTIFIX 73488897 1475623 Dead/Cancelled |
ACME SIGNALISATION INC. 1984-07-09 |
 MULTIFIX 72321364 0881963 Dead/Expired |
BARRETT CHEMICAL COMPANY 1969-03-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.