Pencylcap
Needle, Hypodermic, Single Lumen
B. BRAUN MELSUNGEN AG
The following data is part of a premarket notification filed by B. Braun Melsungen Ag with the FDA for Pencylcap.
Pre-market Notification Details
| Device ID | K152050 |
| 510k Number | K152050 |
| Device Name: | Pencylcap |
| Classification | Needle, Hypodermic, Single Lumen |
| Applicant | B. BRAUN MELSUNGEN AG CARL-BRAUN STRASSE 1 Melsungen, DE 34212 |
| Contact | Hans-ulrich Gaudin |
| Correspondent | Karin Lubbers YES MEDICAL DEVICE SERVICES GMBH BAHNSTRASSE 42-46 Friedrichsdorf, DE 61381 |
| Product Code | FMI |
| CFR Regulation Number | 880.5570 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2015-07-23 |
| Decision Date | 2016-03-02 |
Trademark Results [Pencylcap]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
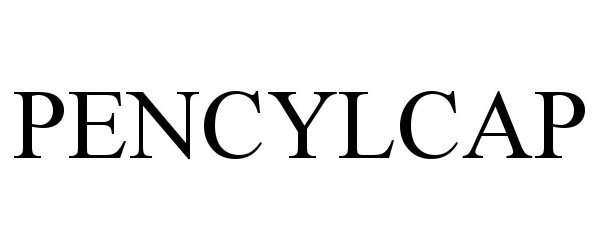 PENCYLCAP 90473829 not registered Live/Pending |
MERCK KGAA 2021-01-19 |
 PENCYLCAP 79135807 4561924 Live/Registered |
Merck KGaA 2013-07-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.