MaxiGen
Filler, Bone Void, Calcium Compound
HANS BIOMED CORP.
The following data is part of a premarket notification filed by Hans Biomed Corp. with the FDA for Maxigen.
Pre-market Notification Details
| Device ID | K152077 |
| 510k Number | K152077 |
| Device Name: | MaxiGen |
| Classification | Filler, Bone Void, Calcium Compound |
| Applicant | HANS BIOMED CORP. 64, YUSEONG-DAERO 1628 BEON-GIL, YUSEONG-GU Daejeon, KR 305-811 |
| Contact | Lucy Choi |
| Correspondent | Patsy J Trisler Trisler Consulting 5600 Wisconsin Ave, #509 Chevy Chase, MD 20815 |
| Product Code | MQV |
| CFR Regulation Number | 888.3045 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2015-07-27 |
| Decision Date | 2016-04-21 |
| Summary: | summary |
Trademark Results [MaxiGen]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
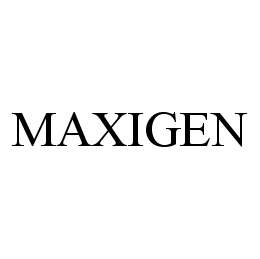 MAXIGEN 78422330 not registered Dead/Abandoned |
Biopharmaceuticals LLC 2004-05-20 |
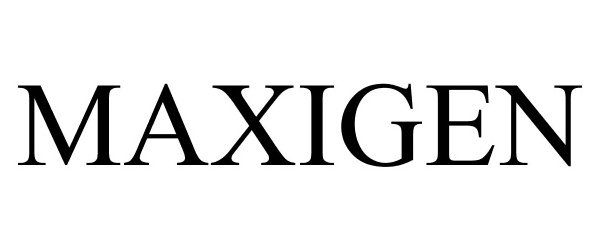 MAXIGEN 77160504 not registered Dead/Abandoned |
Rockmore Industries, LLC 2007-04-19 |
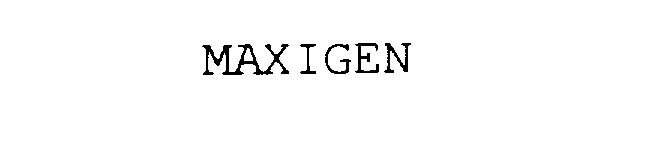 MAXIGEN 75835055 not registered Dead/Abandoned |
James, Jonathan B. 1999-11-14 |
 MAXIGEN 74720887 not registered Dead/Abandoned |
Vanner Weldon, Inc. 1995-08-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.