MAIA
Ophthalmoscope, Laser, Scanning
CENTERVUE SPA
The following data is part of a premarket notification filed by Centervue Spa with the FDA for Maia.
Pre-market Notification Details
| Device ID | K153181 |
| 510k Number | K153181 |
| Device Name: | MAIA |
| Classification | Ophthalmoscope, Laser, Scanning |
| Applicant | CENTERVUE SPA VIA SAN MARCO 9H Padova, IT 35129 |
| Contact | Roberto Gabriotti |
| Correspondent | Roberto Gabriotti CENTERVUE SPA VIA SAN MARCO 9H Padova, IT 35129 |
| Product Code | MYC |
| CFR Regulation Number | 886.1570 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2015-11-03 |
| Decision Date | 2016-06-08 |
| Summary: | summary |
Trademark Results [MAIA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MAIA 98579383 not registered Live/Pending |
Regent Properties, LLC 2024-05-31 |
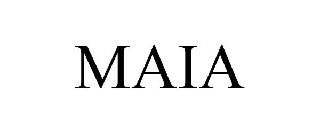 MAIA 98579360 not registered Live/Pending |
Regent Properties, LLC 2024-05-31 |
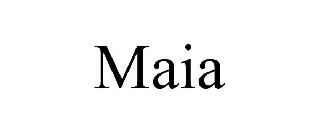 MAIA 98562887 not registered Live/Pending |
W. Kirchhoff Inc. 2024-05-22 |
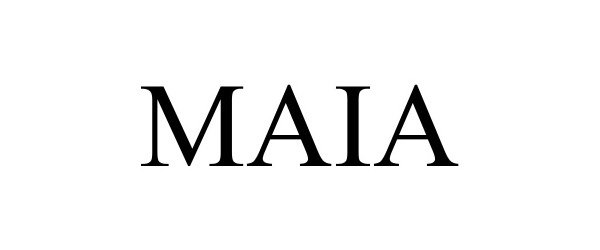 MAIA 98436901 not registered Live/Pending |
P.Q.L., Inc. 2024-03-06 |
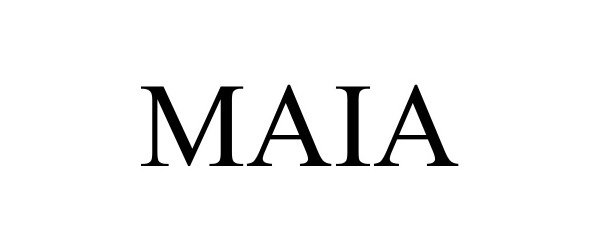 MAIA 98311927 not registered Live/Pending |
Microsoft Corporation 2023-12-13 |
 MAIA 97609548 not registered Live/Pending |
Maia Synergy LLC 2022-09-27 |
 MAIA 97324711 not registered Live/Pending |
Start.io Inc. 2022-03-22 |
 MAIA 97249850 not registered Live/Pending |
Maia Pharmaceuticals, Inc. 2022-02-02 |
 MAIA 97125255 not registered Live/Pending |
E.S. Firefly LLC 2021-11-15 |
 MAIA 90338987 not registered Live/Pending |
Pippy Sips LLC 2020-11-24 |
 MAIA 90228456 not registered Live/Pending |
HOK, SIVMEY 2020-10-01 |
 MAIA 88765092 not registered Live/Pending |
Maia Supplements LLC 2020-01-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.