Strive
Stimulator, Nerve, Transcutaneous, For Pain Relief
DJO, LLC
The following data is part of a premarket notification filed by Djo, Llc with the FDA for Strive.
Pre-market Notification Details
| Device ID | K153704 |
| 510k Number | K153704 |
| Device Name: | Strive |
| Classification | Stimulator, Nerve, Transcutaneous, For Pain Relief |
| Applicant | DJO, LLC 1430 DECISION ST. Vista, CA 92081 |
| Contact | Rand Daoud |
| Correspondent | Rand Daoud DJO, LLC 1430 DECISION ST. Vista, CA 92081 |
| Product Code | GZJ |
| Subsequent Product Code | NUH |
| Subsequent Product Code | NYN |
| CFR Regulation Number | 882.5890 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Abbreviated |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2015-12-23 |
| Decision Date | 2016-06-03 |
| Summary: | summary |
Trademark Results [Strive]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 STRIVE 98688283 not registered Live/Pending |
BRAND CONTROL LTD 2024-08-08 |
 STRIVE 98491324 not registered Live/Pending |
Strive Nutrition Corp. 2024-04-09 |
 STRIVE 98197666 not registered Live/Pending |
Hive Cocktail, Inc. 2023-09-26 |
 STRIVE 98090425 not registered Live/Pending |
Embecta Corp. 2023-07-18 |
 STRIVE 98015704 not registered Live/Pending |
Universal Engineering Sciences, LLC 2023-05-26 |
 STRIVE 97852721 not registered Live/Pending |
Olavarria, Angel L 2023-03-23 |
 STRIVE 97689224 not registered Live/Pending |
STRIVE SOFTWARE 2022-11-22 |
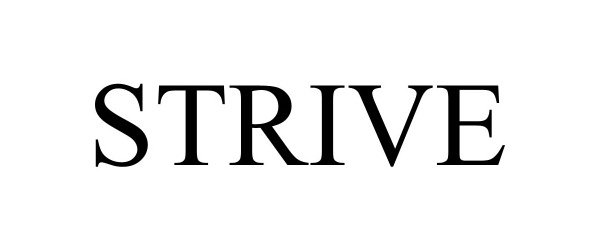 STRIVE 97689198 not registered Live/Pending |
STRIVE SOFTWARE 2022-11-22 |
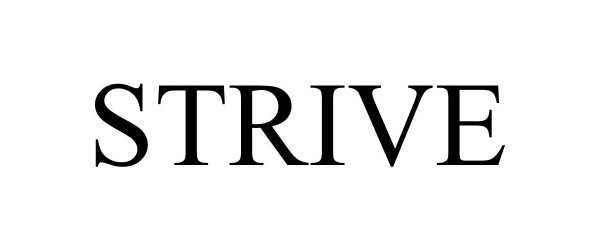 STRIVE 97469362 not registered Live/Pending |
CIRCLE SPECIALTY INC. 2022-06-21 |
 STRIVE 97437851 not registered Live/Pending |
Strive Enterprises, Inc. 2022-06-01 |
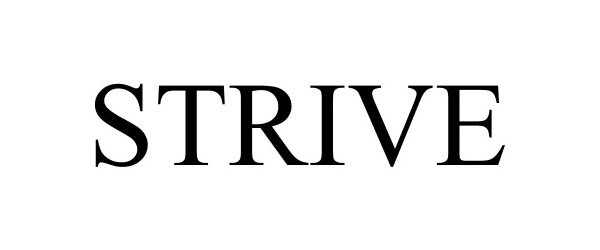 STRIVE 97426379 not registered Live/Pending |
Strive Enterprises, Inc. 2022-05-24 |
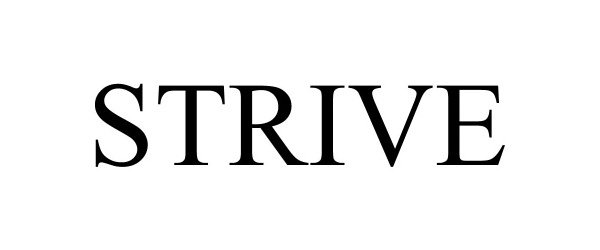 STRIVE 97398960 not registered Live/Pending |
Strive Creations, LLC 2022-05-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.