OSSIX PLUS
Barrier, Animal Source, Intraoral
Datum Dental Ltd.
The following data is part of a premarket notification filed by Datum Dental Ltd. with the FDA for Ossix Plus.
Pre-market Notification Details
| Device ID | K160281 |
| 510k Number | K160281 |
| Device Name: | OSSIX PLUS |
| Classification | Barrier, Animal Source, Intraoral |
| Applicant | Datum Dental Ltd. 1 Bat Sheva St., PO Box 6170 Lod, IL 7116003 |
| Contact | Arie Goldlust |
| Correspondent | Janice M. Hogan Hogan Lovells US LLP 1835 Market Street, 29th Floor Philadelphia, PA 19103 |
| Product Code | NPL |
| CFR Regulation Number | 872.3930 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2016-02-02 |
| Decision Date | 2016-08-04 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 07290015477765 | K160281 | 000 |
| 07290015477727 | K160281 | 000 |
| 07290015477710 | K160281 | 000 |
Trademark Results [OSSIX PLUS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
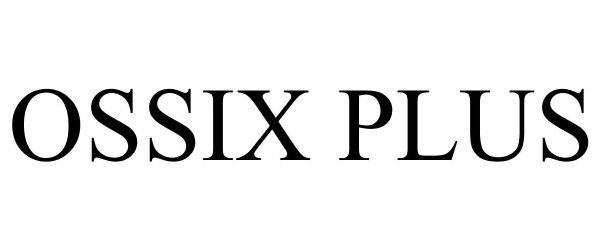 OSSIX PLUS 77336205 not registered Dead/Abandoned |
Johnson & Johnson 2007-11-25 |
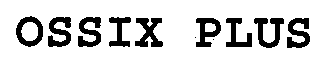 OSSIX PLUS 76686242 3649127 Live/Registered |
DATUM BIOTECH LTD. 2008-01-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.