AGNES
Electrosurgical, Cutting & Coagulation & Accessories
GOWOONSESANG COSMETICS CO., LTD
The following data is part of a premarket notification filed by Gowoonsesang Cosmetics Co., Ltd with the FDA for Agnes.
Pre-market Notification Details
| Device ID | K160469 |
| 510k Number | K160469 |
| Device Name: | AGNES |
| Classification | Electrosurgical, Cutting & Coagulation & Accessories |
| Applicant | GOWOONSESANG COSMETICS CO., LTD (Seohyeon-dong, 4, 5F Cocoplaza), 20, Seohyeon-ro 210beon-gil, Bundang-gu Seongnam-si, KR 463-824 |
| Contact | Kyeong Tai Ko |
| Correspondent | Dongha Lee KMC, Inc. Room No. 409, ACE Technotower 1-Cha, 38-9, Digital-ro 31-gil, Guro-gu Seoul, KR 152-766 |
| Product Code | GEI |
| CFR Regulation Number | 878.4400 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2016-02-19 |
| Decision Date | 2017-01-05 |
| Summary: | summary |
Trademark Results [AGNES]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
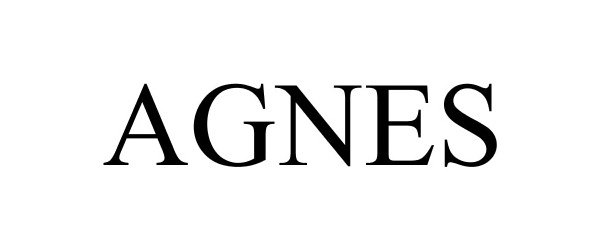 AGNES 88476473 5949738 Live/Registered |
Lindsey Adelman Studio LLC 2019-06-17 |
 AGNES 88346432 5895046 Live/Registered |
AMD Global Telemedicine, Inc. 2019-03-19 |
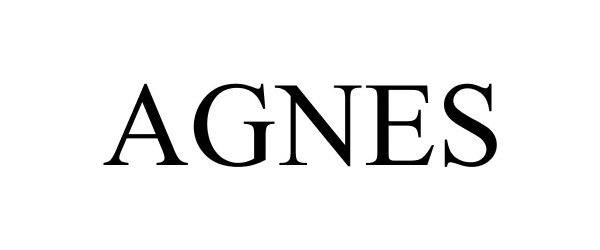 AGNES 86834011 5080576 Live/Registered |
AGNES MEDICAL CO., LTD. 2015-11-30 |
 AGNES 86069496 not registered Dead/Abandoned |
Del Boca, Adrian, M.D., P.A. 2013-09-19 |
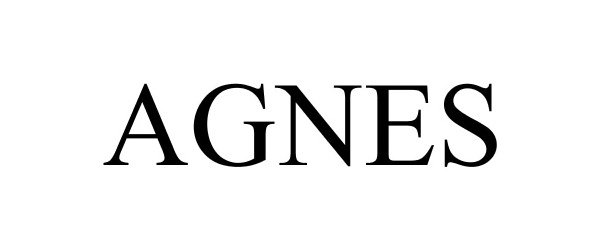 AGNES 85957733 4509648 Live/Registered |
AMD Global Telemedicine 2013-06-12 |
 AGNES 77833633 not registered Dead/Abandoned |
Del Boca, Adrian, M.D., P.A. 2009-09-23 |
 AGNES 73454430 1301909 Dead/Cancelled |
CBS Inc. 1983-11-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.