VisualEyes
Nystagmograph
Interacoustics A/S
The following data is part of a premarket notification filed by Interacoustics A/s with the FDA for Visualeyes.
Pre-market Notification Details
| Device ID | K163149 |
| 510k Number | K163149 |
| Device Name: | VisualEyes |
| Classification | Nystagmograph |
| Applicant | Interacoustics A/S Audiometer Alle 1 Middelfart, DK 5500 |
| Contact | Erik Nielsen |
| Correspondent | Erik Nielsen Interacoustics A/S Audiometer Alle 1 Middelfart, DK 5500 |
| Product Code | GWN |
| CFR Regulation Number | 882.1460 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2016-11-09 |
| Decision Date | 2017-04-26 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 05711117195989 | K163149 | 000 |
Trademark Results [VisualEyes]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
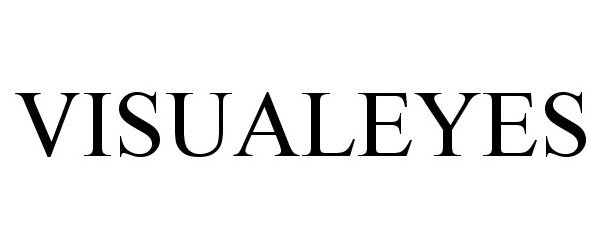 VISUALEYES 86372343 not registered Dead/Abandoned |
HTC Corporation 2014-08-20 |
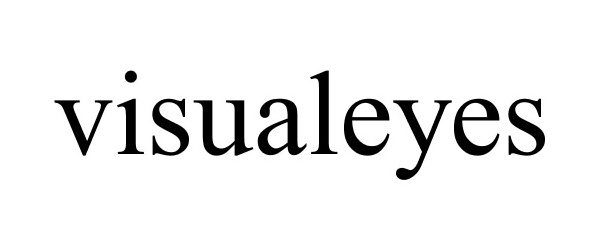 VISUALEYES 86336136 4688699 Live/Registered |
OPTICAL CONNECTION INTERNATIONAL CORPORATION 2014-07-14 |
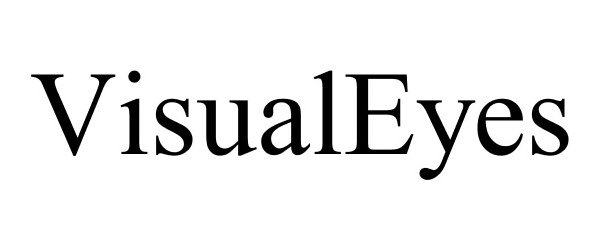 VISUALEYES 86144025 4568372 Live/Registered |
M.Labs, Inc. 2013-12-15 |
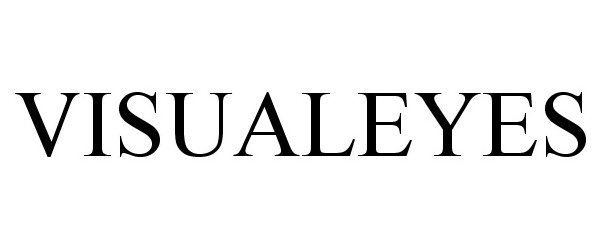 VISUALEYES 86086006 4639689 Live/Registered |
Make Money Limited 2013-10-08 |
 VISUALEYES 79006377 3073735 Dead/Cancelled |
Intelligent Puzzles Australia Pty Ltd ACN 006 801 654 2004-10-18 |
 VISUALEYES 77625597 not registered Dead/Abandoned |
Visualeyes AB 2008-12-03 |
 VISUALEYES 75309703 not registered Dead/Abandoned |
HARRIS CORPORATION 1997-06-16 |
 VISUALEYES 74090587 1679299 Dead/Cancelled |
Visitec Company 1990-08-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.