EXPRT™ Revision Hip System - EXPRT™ Hip Distal Stem, EXPRT™ Hip Standard Offset Proximal Body Implant, EXPRT™ Hip Lateral Offset Proximal Body Implant, EXPRT™ Hip Capture Bolt
Prosthesis, Hip, Constrained, Cemented Or Uncemented, Metal/polymer
ENCORE MEDICAL, L.P.
The following data is part of a premarket notification filed by Encore Medical, L.p. with the FDA for Exprt™ Revision Hip System - Exprt™ Hip Distal Stem, Exprt™ Hip Standard Offset Proximal Body Implant, Exprt™ Hip Lateral Offset Proximal Body Implant, Exprt™ Hip Capture Bolt.
Pre-market Notification Details
| Device ID | K163497 |
| 510k Number | K163497 |
| Device Name: | EXPRT™ Revision Hip System - EXPRT™ Hip Distal Stem, EXPRT™ Hip Standard Offset Proximal Body Implant, EXPRT™ Hip Lateral Offset Proximal Body Implant, EXPRT™ Hip Capture Bolt |
| Classification | Prosthesis, Hip, Constrained, Cemented Or Uncemented, Metal/polymer |
| Applicant | ENCORE MEDICAL, L.P. 9800 METRIC BLVD. Austin, TX 78758 |
| Contact | Teffany Hutto |
| Correspondent | Teffany Hutto ENCORE MEDICAL, L.P. 9800 METRIC BLVD. Austin, TX 78758 |
| Product Code | KWZ |
| Subsequent Product Code | LWJ |
| Subsequent Product Code | LZO |
| CFR Regulation Number | 888.3310 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2016-12-13 |
| Decision Date | 2017-03-02 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 00190446324188 | K163497 | 000 |
| 00190446324171 | K163497 | 000 |
| 00190446324164 | K163497 | 000 |
| 00190446153962 | K163497 | 000 |
| 00190446153955 | K163497 | 000 |
| 00190446153948 | K163497 | 000 |
| 00190446153931 | K163497 | 000 |
| 00190446153924 | K163497 | 000 |
| 00190446153917 | K163497 | 000 |
Trademark Results [EXPRT]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
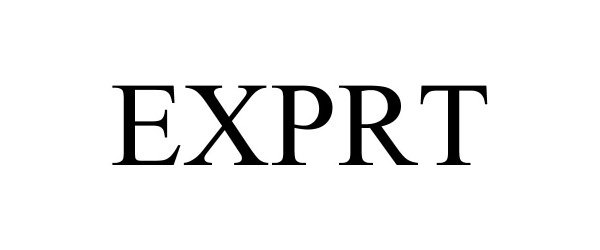 EXPRT 88693932 not registered Live/Pending |
Silva, Jenat M. 2019-11-15 |
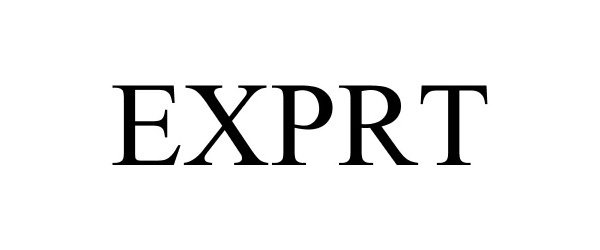 EXPRT 87753910 not registered Dead/Abandoned |
Silva, Jenat M. 2018-01-12 |
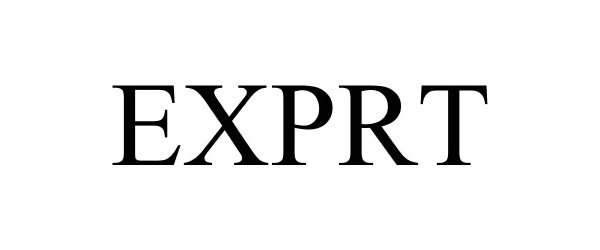 EXPRT 86594246 4862686 Live/Registered |
Encore Medical, L.P. 2015-04-10 |
 EXPRT 76496992 2805963 Dead/Cancelled |
Icon Advertising & Design, Inc. 2003-03-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.