Bilicocoon™ Phototherapy System
Unit, Neonatal Phototherapy
NEOMEDLIGHT
The following data is part of a premarket notification filed by Neomedlight with the FDA for Bilicocoon™ Phototherapy System.
Pre-market Notification Details
| Device ID | K163526 |
| 510k Number | K163526 |
| Device Name: | Bilicocoon™ Phototherapy System |
| Classification | Unit, Neonatal Phototherapy |
| Applicant | NEOMEDLIGHT 89-90 Rue Frederic FAYS Villeurbanne, FR 69100 |
| Contact | Pierre Saint Girons |
| Correspondent | Pierre Saint Girons NEOMEDLIGHT 89-90 Rue Frederic FAYS Villeurbanne, FR 69100 |
| Product Code | LBI |
| CFR Regulation Number | 880.5700 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2016-12-16 |
| Decision Date | 2017-10-06 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 03760012570119 | K163526 | 000 |
| 03760012570096 | K163526 | 000 |
| 03760012570065 | K163526 | 000 |
| 03760012570058 | K163526 | 000 |
| 03760012570027 | K163526 | 000 |
| 03760012570010 | K163526 | 000 |
| 03760012570003 | K163526 | 000 |
Trademark Results [Bilicocoon]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
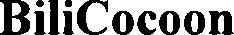 BILICOCOON 79348352 not registered Live/Pending |
NeoMedLight 2022-08-01 |
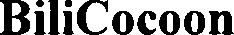 BILICOCOON 79181064 5038567 Live/Registered |
NeoMedLight 2015-09-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.