Halcyon
Accelerator, Linear, Medical
Varian Medical Systems Inc.
The following data is part of a premarket notification filed by Varian Medical Systems Inc. with the FDA for Halcyon.
Pre-market Notification Details
| Device ID | K170817 |
| 510k Number | K170817 |
| Device Name: | Halcyon |
| Classification | Accelerator, Linear, Medical |
| Applicant | Varian Medical Systems Inc. 911 Hansen Way Palo Alto, CA 94304 |
| Contact | Peter J. Coronado |
| Correspondent | Peter J. Coronado Varian Medical Systems Inc. 911 Hansen Way Palo Alto, CA 94304 |
| Product Code | IYE |
| CFR Regulation Number | 892.5050 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2017-03-20 |
| Decision Date | 2017-06-27 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 00857235006006 | K170817 | 000 |
Trademark Results [Halcyon]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HALCYON 98780313 not registered Live/Pending |
Batesville Casket Company, LLC 2024-10-01 |
 HALCYON 98742572 not registered Live/Pending |
Shenzhen Heshengyuan Hardware Co., Ltd. 2024-09-10 |
 HALCYON 98735393 not registered Live/Pending |
Plant Food Systems, Inc. 2024-09-05 |
 HALCYON 98617602 not registered Live/Pending |
Lauren Mia Music LLC 2024-06-25 |
 HALCYON 98511856 not registered Live/Pending |
Halcyon Tech, Inc. 2024-04-22 |
 HALCYON 98496577 not registered Live/Pending |
Paragon Motorcycles Inc. 2024-04-12 |
 HALCYON 98447124 not registered Live/Pending |
Halcyon Carbon, Inc. 2024-03-13 |
 HALCYON 98312621 not registered Live/Pending |
BJ2, LLC 2023-12-13 |
 HALCYON 98239615 not registered Live/Pending |
FCA US LLC 2023-10-25 |
 HALCYON 98224834 not registered Live/Pending |
Halcyon Tech, Inc. 2023-10-16 |
 HALCYON 97630400 not registered Live/Pending |
TotalBoat, LLC 2022-10-13 |
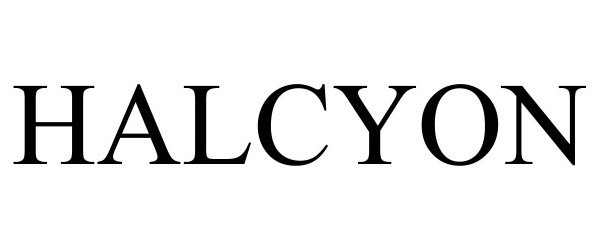 HALCYON 97447612 not registered Live/Pending |
The Halcyon Movement 2022-06-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.