Silk'n Infinity
Light Based Over-the-counter Hair Removal
Home Skinovations Ltd.
The following data is part of a premarket notification filed by Home Skinovations Ltd. with the FDA for Silk'n Infinity.
Pre-market Notification Details
| Device ID | K171433 |
| 510k Number | K171433 |
| Device Name: | Silk'n Infinity |
| Classification | Light Based Over-the-counter Hair Removal |
| Applicant | Home Skinovations Ltd. Tabor Building, Shaar Yokneam Yoqneam Illit, IL |
| Contact | Amit Goren |
| Correspondent | Amit Goren A. Stein - Regulatory Affairs Consulting Ltd. 20 Hata'as Str., Suite 102 Kfar Saba, IL |
| Product Code | OHT |
| Subsequent Product Code | NFO |
| Subsequent Product Code | ONF |
| CFR Regulation Number | 878.4810 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2017-05-15 |
| Decision Date | 2017-08-11 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 17290015764756 | K171433 | 000 |
| 27290019840255 | K171433 | 000 |
| 27290019840248 | K171433 | 000 |
Trademark Results [Silk'n Infinity]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
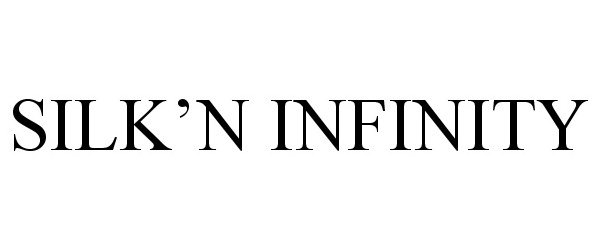 SILK'N INFINITY 86910868 5393885 Live/Registered |
Home Skinovations Ltd. 2016-02-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.