Easyscreen
Audiometer
Maico Diagnostics GmbH
The following data is part of a premarket notification filed by Maico Diagnostics Gmbh with the FDA for Easyscreen.
Pre-market Notification Details
| Device ID | K171506 |
| 510k Number | K171506 |
| Device Name: | Easyscreen |
| Classification | Audiometer |
| Applicant | Maico Diagnostics GmbH Sickingenstr. 70-71 Berlin, DE 10553 |
| Contact | Amy Yanta |
| Correspondent | Amy Yanta Maico Diagnostics GmbH Sickingenstr. 70-71 Berlin, DE 10553 |
| Product Code | EWO |
| CFR Regulation Number | 874.1050 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2017-05-23 |
| Decision Date | 2017-08-29 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 04260176121169 | K171506 | 000 |
Trademark Results [Easyscreen]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
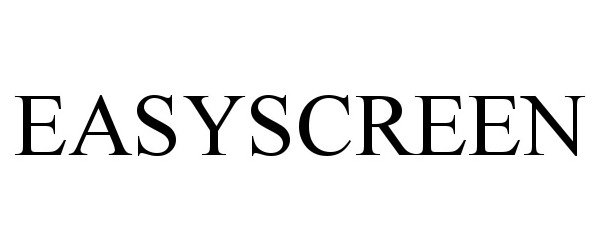 EASYSCREEN 98505769 not registered Live/Pending |
Sunset Products Limited Partnership 2024-04-17 |
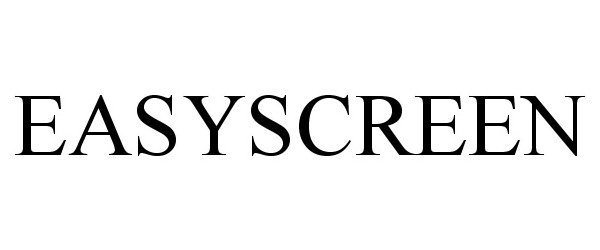 EASYSCREEN 85537293 4639209 Live/Registered |
Callum Bush 2012-02-08 |
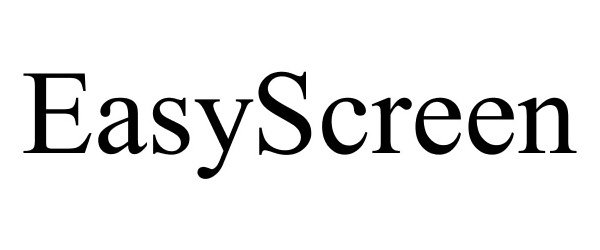 EASYSCREEN 85325453 4211013 Live/Registered |
Nuvasive Inc. 2011-05-19 |
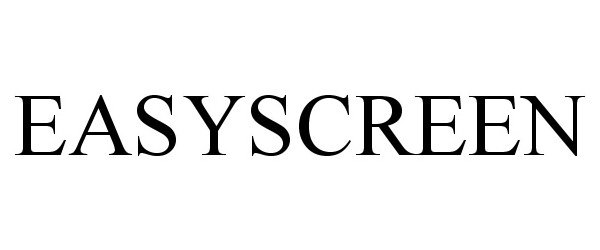 EASYSCREEN 85163837 4281345 Dead/Cancelled |
MAREX SPECTRON GROUP LIMITED 2010-10-28 |
 EASYSCREEN 79391120 not registered Live/Pending |
Genetic Signatures Limited 2024-02-14 |
 EASYSCREEN 79345602 not registered Live/Pending |
Genetic Signatures Limited 2021-08-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.