NAJA™ Ligament Correction System
Bone Fixation Cerclage, Sublaminar
Cousin Biotech S.A.S.
The following data is part of a premarket notification filed by Cousin Biotech S.a.s. with the FDA for Naja™ Ligament Correction System.
Pre-market Notification Details
| Device ID | K172206 |
| 510k Number | K172206 |
| Device Name: | NAJA™ Ligament Correction System |
| Classification | Bone Fixation Cerclage, Sublaminar |
| Applicant | Cousin Biotech S.A.S. 8 Rue De L'abbe Bonpain Wervicq-sud, FR 59117 |
| Contact | Mathilde Collet |
| Correspondent | Franck Pelletier Cousin Biotech S.A.S. 8 Rue De L'abbe Bonpain Wervicq-sud, FR 59117 |
| Product Code | OWI |
| CFR Regulation Number | 888.3010 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2017-07-21 |
| Decision Date | 2018-04-10 |
| Summary: | summary |
Trademark Results [NAJA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NAJA 97244135 not registered Live/Pending |
BIG BANG 2022-01-28 |
 NAJA 90578082 not registered Live/Pending |
Foshan Tuorun Precision Hardware Technology Co., Ltd. 2021-03-14 |
 NAJA 87674407 not registered Dead/Abandoned |
ROCKWOOD & HINES GLASS GROUP CO. Ltd 2017-11-07 |
 NAJA 86047789 4739834 Live/Registered |
BLACK CASHMERE LABS, INC. 2013-08-26 |
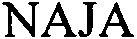 NAJA 79164756 4883650 Live/Registered |
COUSIN BIOTECH 2015-03-18 |
 NAJA 78575782 3110572 Dead/Cancelled |
Mayhew, Donald R. 2005-02-26 |
 NAJA 77333472 3530129 Dead/Cancelled |
IFILL, KERWIN 2007-11-19 |
 NAJA 77109786 3328680 Dead/Cancelled |
Native American Journalists Association 2007-02-16 |
 NAJA 76100059 2661477 Dead/Cancelled |
NAJA S.A. 2000-07-31 |
 NAJA 73625356 1577685 Dead/Cancelled |
EXAEQUO SA 1986-10-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.