B-ONE® Total Hip System
Prosthesis, Hip, Semi-constrained, Metal/ceramic/polymer, Cemented Or Non-porous, Uncemented
B-One Ortho Corp.
The following data is part of a premarket notification filed by B-one Ortho Corp. with the FDA for B-one® Total Hip System.
Pre-market Notification Details
| Device ID | K173380 |
| 510k Number | K173380 |
| Device Name: | B-ONE® Total Hip System |
| Classification | Prosthesis, Hip, Semi-constrained, Metal/ceramic/polymer, Cemented Or Non-porous, Uncemented |
| Applicant | b-One Ortho Corp. 3 Wing Drive Suite #259 Cedar Knolls, NJ 07927 |
| Contact | Clark Hutton |
| Correspondent | Allison Geick Lakeshore Medical Device Consulting LLC 3 Wing Drive Suite 259 Cedar Knolls, NJ 07927 |
| Product Code | LZO |
| Subsequent Product Code | HWC |
| Subsequent Product Code | MEH |
| CFR Regulation Number | 888.3353 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2017-10-30 |
| Decision Date | 2018-08-30 |
| Summary: | summary |
Trademark Results [B-ONE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
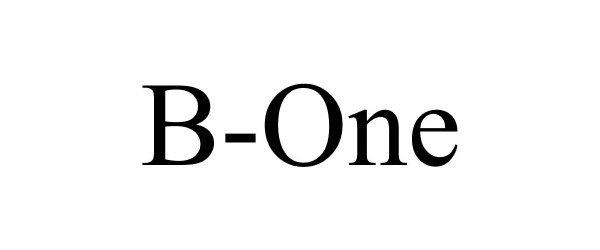 B-ONE 88564003 not registered Live/Pending |
Kura Biotec SpA 2019-08-02 |
 B-ONE 87137464 5868751 Live/Registered |
B-One Ortho, Inc. 2016-08-12 |
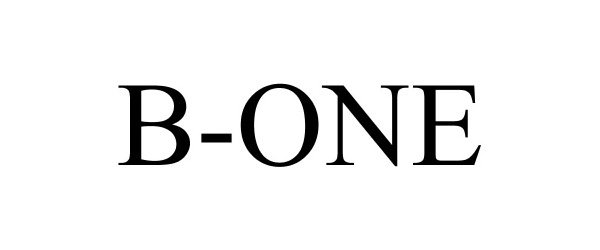 B-ONE 87137456 5868749 Live/Registered |
B-One Ortho, Inc. 2016-08-12 |
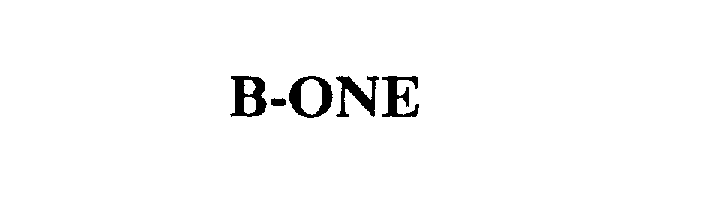 B-ONE 75585296 2311332 Dead/Cancelled |
HUFFY CORPORATION 1998-11-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.