ELEOS™ Bipolar Acetabular System
Prosthesis, Hip, Hemi-, Femoral, Metal/polymer, Cemented Or Uncemented
Onkos Surgical, Inc.
The following data is part of a premarket notification filed by Onkos Surgical, Inc. with the FDA for Eleos™ Bipolar Acetabular System.
Pre-market Notification Details
| Device ID | K180130 |
| 510k Number | K180130 |
| Device Name: | ELEOS™ Bipolar Acetabular System |
| Classification | Prosthesis, Hip, Hemi-, Femoral, Metal/polymer, Cemented Or Uncemented |
| Applicant | Onkos Surgical, Inc. 77 East Halsey Road Parsippany, NJ 07054 |
| Contact | Jan Triani |
| Correspondent | Jan Triani Onkos Surgical, Inc. 77 East Halsey Road Parsippany, NJ 07054 |
| Product Code | KWY |
| CFR Regulation Number | 888.3390 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2018-01-17 |
| Decision Date | 2018-02-23 |
| Summary: | summary |
Trademark Results [ELEOS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ELEOS 98863077 not registered Live/Pending |
Eleos Pharmaceuticals, Inc. 2024-11-20 |
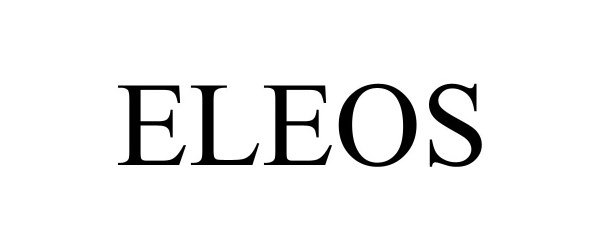 ELEOS 98671529 not registered Live/Pending |
Eleos Mental Systems Ltd. 2024-07-29 |
 ELEOS 98259952 not registered Live/Pending |
VidhcoShop LLC 2023-11-08 |
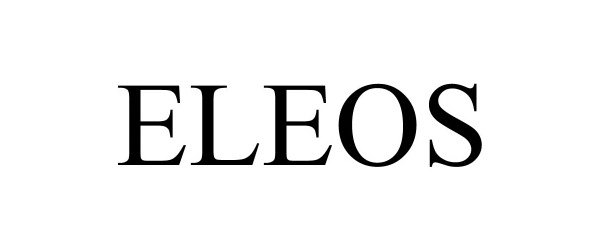 ELEOS 97280289 not registered Live/Pending |
A. FLOROS IMPORTS, LLC 2022-02-23 |
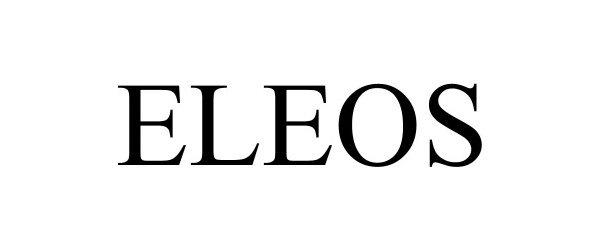 ELEOS 97267783 not registered Live/Pending |
Aicor Inc. 2022-02-15 |
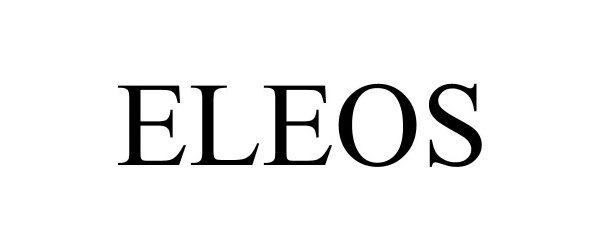 ELEOS 90738659 not registered Live/Pending |
Eleos Pharmaceuticals, LLC 2021-05-27 |
 ELEOS 90138174 not registered Live/Pending |
Adams, Adria Nicole 2020-08-26 |
 ELEOS 90032470 not registered Live/Pending |
Laserware Ltd 2020-07-02 |
 ELEOS 88912164 not registered Live/Pending |
Eleos Technologies, LLC 2020-05-12 |
 ELEOS 88912082 not registered Live/Pending |
Eleos Technologies, LLC 2020-05-12 |
 ELEOS 88907392 not registered Live/Pending |
Eleos Technologies, LLC 2020-05-08 |
 ELEOS 87336423 not registered Dead/Abandoned |
Onkos Surgical, Inc. 2017-02-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.