Transpara
Radiological Computer Assisted Detection/diagnosis Software For Lesions Suspicious For Cancer
ScreenPoint Medical BV
The following data is part of a premarket notification filed by Screenpoint Medical Bv with the FDA for Transpara.
Pre-market Notification Details
| Device ID | K181704 |
| 510k Number | K181704 |
| Device Name: | Transpara |
| Classification | Radiological Computer Assisted Detection/diagnosis Software For Lesions Suspicious For Cancer |
| Applicant | ScreenPoint Medical BV Stationsplein 26 Nijmegen, NL 6512ab |
| Contact | Nico Karssemeijer |
| Correspondent | Nico Karssemeijer ScreenPoint Medical BV Stationsplein 26 Nijmegen, NL 6512ab |
| Product Code | QDQ |
| CFR Regulation Number | 892.2090 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2018-06-27 |
| Decision Date | 2018-11-21 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 08719326450100 | K181704 | 000 |
Trademark Results [Transpara]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
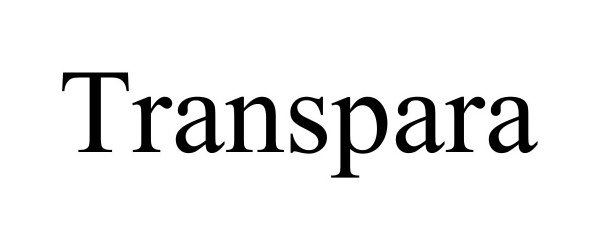 TRANSPARA 97560177 not registered Live/Pending |
CPS Technology Holdings LLC 2022-08-23 |
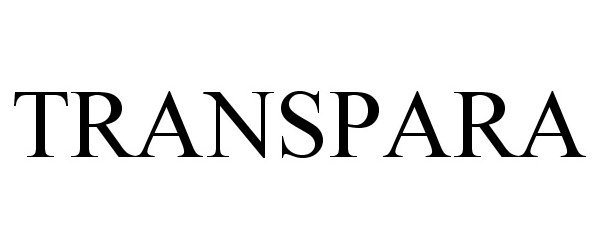 TRANSPARA 87822676 5906201 Live/Registered |
Transpara, LLC 2018-03-06 |
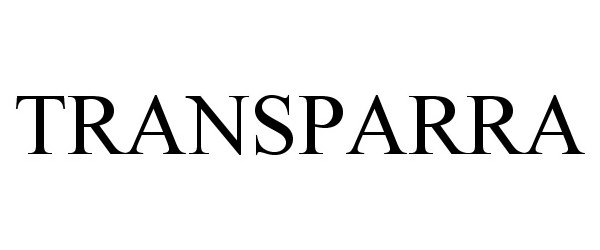 TRANSPARA 87012066 not registered Dead/Abandoned |
C.R. Laurence Co., Inc. 2016-04-24 |
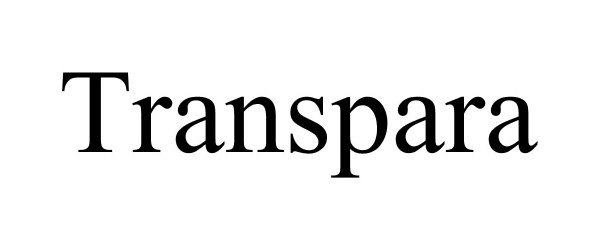 TRANSPARA 86756857 4968025 Live/Registered |
ScreenPoint Medical BV 2015-09-15 |
 TRANSPARA 77818070 not registered Dead/Abandoned |
Matakina Technology Limited 2009-09-02 |
 TRANSPARA 71592472 0541135 Dead/Expired |
BURLINGTON MILLS CORPORATION 1950-02-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.