FFRct
Coronary Vascular Physiologic Simulation Software
HeartFlow, Inc
The following data is part of a premarket notification filed by Heartflow, Inc with the FDA for Ffrct.
Pre-market Notification Details
| Device ID | K182035 |
| 510k Number | K182035 |
| Device Name: | FFRct |
| Classification | Coronary Vascular Physiologic Simulation Software |
| Applicant | HeartFlow, Inc 1400 Seaport Boulevard Building B Redwood City, CA 94063 |
| Contact | Windi Hary |
| Correspondent | Windi Hary HeartFlow, Inc 1400 Seaport Boulevard Building B Redwood City, CA 94063 |
| Product Code | PJA |
| CFR Regulation Number | 870.1415 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Special |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2018-07-30 |
| Decision Date | 2018-12-06 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 00853341006022 | K182035 | 000 |
Trademark Results [FFRct]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
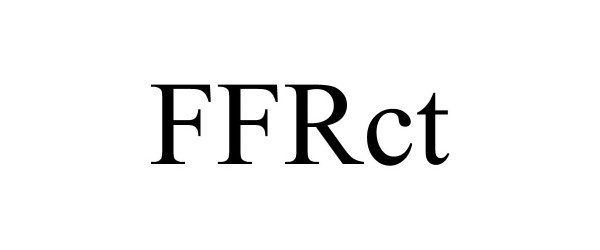 FFRCT 90808627 not registered Live/Pending |
Elucid Bioimaging Inc. 2021-07-02 |
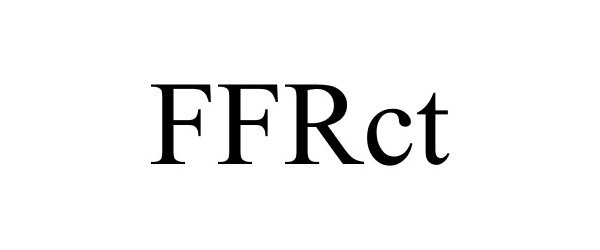 FFRCT 85334348 not registered Dead/Abandoned |
Heartflow, Inc. 2011-05-31 |
 FFRCT 85284255 4641484 Dead/Cancelled |
Heartflow, Inc. 2011-04-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.