SP-LINK™ System
Spinous Process Plate
Medical Designs LLC
The following data is part of a premarket notification filed by Medical Designs Llc with the FDA for Sp-link™ System.
Pre-market Notification Details
| Device ID | K182352 |
| 510k Number | K182352 |
| Device Name: | SP-LINK™ System |
| Classification | Spinous Process Plate |
| Applicant | Medical Designs LLC 6709 S. Minnesota Ave, Suite 204 Sioux Falls, SD 57108 |
| Contact | Kristi Vondra |
| Correspondent | Kristi Vondra Medical Designs LLC 6709 S. Minnesota Ave, Suite 204 Sioux Falls, SD 57108 |
| Product Code | PEK |
| CFR Regulation Number | 888.3050 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2018-08-29 |
| Decision Date | 2018-11-21 |
| Summary: | summary |
Trademark Results [SP-LINK]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SP-LINK 88833410 not registered Live/Pending |
Asfora IP, LLC 2020-03-13 |
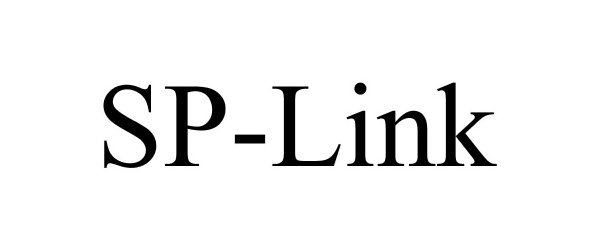 SP-LINK 86922890 not registered Live/Pending |
Asfora IP, LLC 2016-02-29 |
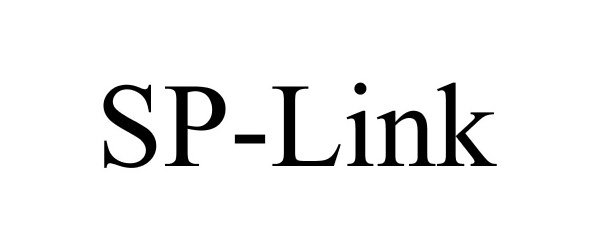 SP-LINK 85839585 not registered Dead/Abandoned |
VibraNova Corporation 2013-02-04 |
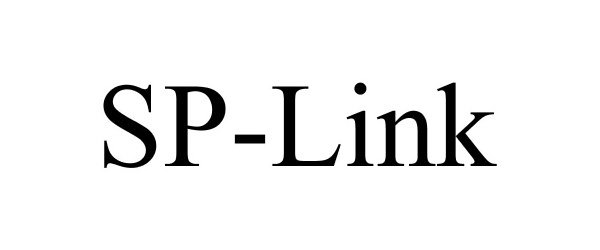 SP-LINK 77781646 not registered Dead/Abandoned |
VibraNova Corporation 2009-07-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.