SI-Restore® Sacroiliac Joint Fixation System
Sacroiliac Joint Fixation
Biofusion Medical
The following data is part of a premarket notification filed by Biofusion Medical with the FDA for Si-restore® Sacroiliac Joint Fixation System.
Pre-market Notification Details
| Device ID | K182919 |
| 510k Number | K182919 |
| Device Name: | SI-Restore® Sacroiliac Joint Fixation System |
| Classification | Sacroiliac Joint Fixation |
| Applicant | Biofusion Medical 2503 Cedarview Drive Austin, TX 78704 |
| Contact | Rylan Reed |
| Correspondent | Daniel Lanois SurgOp Support 7512 Lancaster Gate Frisco, TX 75035 |
| Product Code | OUR |
| CFR Regulation Number | 888.3040 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2018-10-19 |
| Decision Date | 2019-01-25 |
| Summary: | summary |
NIH GUDID Devices
Trademark Results [SI-Restore]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
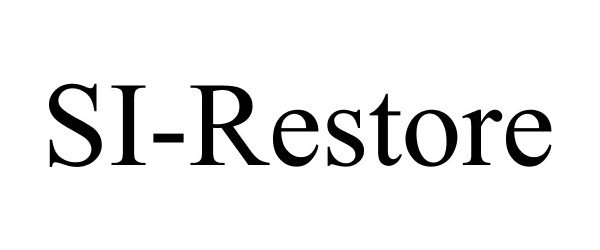 SI-RESTORE 87849969 5747103 Live/Registered |
Bio Fusion Medical 2018-03-26 |
 SI-RESTORE 87849933 5747100 Live/Registered |
Bio Fusion Medical 2018-03-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.