VADER® One Pedicle System MIS And LightMore® Pedicle System 6.0
Thoracolumbosacral Pedicle Screw System
Icotec Ag
The following data is part of a premarket notification filed by Icotec Ag with the FDA for Vader® One Pedicle System Mis And Lightmore® Pedicle System 6.0.
Pre-market Notification Details
| Device ID | K190545 |
| 510k Number | K190545 |
| Device Name: | VADER® One Pedicle System MIS And LightMore® Pedicle System 6.0 |
| Classification | Thoracolumbosacral Pedicle Screw System |
| Applicant | icotec Ag Industriestrasse 12 9450 Altstaetten, CH |
| Contact | Marina Hess |
| Correspondent | Justin Eggleton Musculosketal Clinical Regulatory Affairs 1050 K Street NW, Suite 1000 Washington, DC 20001 |
| Product Code | NKB |
| CFR Regulation Number | 888.3070 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2019-03-04 |
| Decision Date | 2019-06-20 |
Trademark Results [VADER]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
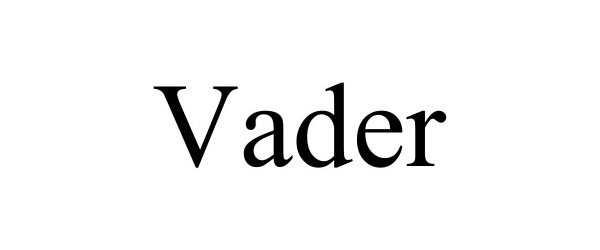 VADER 97865078 not registered Live/Pending |
Tehrani Corp 2023-03-30 |
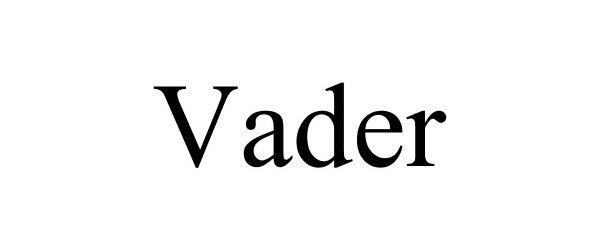 VADER 87728161 5577344 Live/Registered |
Seattle Glove, Inc. 2017-12-20 |
 VADER 86837346 5248513 Live/Registered |
Kiesel Guitars 2015-12-02 |
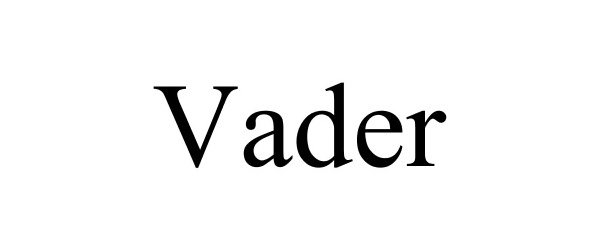 VADER 86781563 not registered Dead/Abandoned |
Clarke, Devrol 2015-10-08 |
 VADER 85951453 not registered Dead/Abandoned |
Soden, Brad 2013-06-05 |
 VADER 85762054 4353734 Live/Registered |
Gilgamesh Brewing, LLC, 2012-10-24 |
 VADER 79183204 5084350 Live/Registered |
icotec AG 2016-01-12 |
 VADER 78488821 3720037 Dead/Cancelled |
Lucasfilm Entertainment Company Ltd. 2004-09-23 |
 VADER 78488812 3794989 Live/Registered |
Lucasfilm Ltd. LLC 2004-09-23 |
 VADER 77221784 3506178 Live/Registered |
Loveland Products, Inc. 2007-07-03 |
 VADER 75933306 not registered Dead/Abandoned |
TEMCO JAPAN CO., LTD. 2000-03-02 |
 VADER 75724308 not registered Dead/Abandoned |
GDH Management, Inc. 1999-06-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.