MCare®Powder-free Nitrile Blue Examination Gloves With Chemotherapy Drugs Labeling Claimed
Polymer Patient Examination Glove
Mercator Medical (Thailand) LTD.
The following data is part of a premarket notification filed by Mercator Medical (thailand) Ltd. with the FDA for Mcare®powder-free Nitrile Blue Examination Gloves With Chemotherapy Drugs Labeling Claimed.
Pre-market Notification Details
| Device ID | K190876 |
| 510k Number | K190876 |
| Device Name: | MCare®Powder-free Nitrile Blue Examination Gloves With Chemotherapy Drugs Labeling Claimed |
| Classification | Polymer Patient Examination Glove |
| Applicant | Mercator Medical (Thailand) LTD. 88/8, 88/9 Moo 12 Tambon Kampaengphet Amphur Rattaphum, TH 90180 |
| Contact | Dariusz Jan Krezymon |
| Correspondent | Kevin Walls Regulatory Insight, Inc. 33 Golden Eagle Lane Littleton, CO 80127 |
| Product Code | LZA |
| CFR Regulation Number | 880.6250 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2019-04-04 |
| Decision Date | 2019-12-02 |
Trademark Results [MCare]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
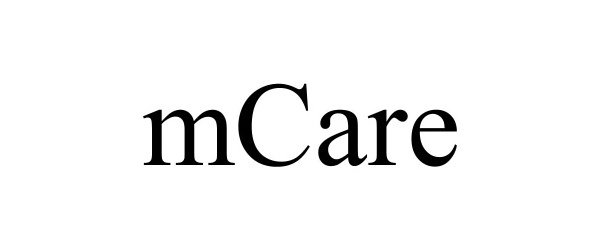 MCARE 97789732 not registered Live/Pending |
Medicus IT, LLC 2023-02-10 |
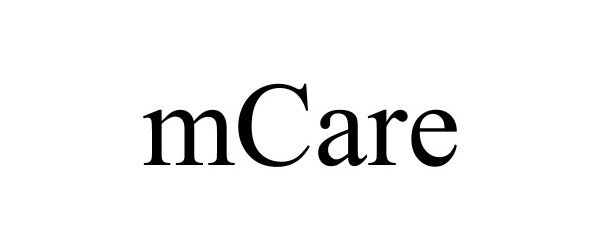 MCARE 90608606 not registered Live/Pending |
Tongshuai Lei 2021-03-29 |
 MCARE 90511708 not registered Live/Pending |
ALSAADI, ABDULRAHMAN 2021-02-04 |
 MCARE 77869278 3929657 Live/Registered |
Mannington Mills, Inc. 2009-11-10 |
 MCARE 77754607 3736582 Live/Registered |
Mercadien Technologies, LLC 2009-06-08 |
 MCARE 77188079 not registered Dead/Abandoned |
E.King Group 2007-05-23 |
 MCARE 76587591 3171875 Live/Registered |
Mannington Carpets, Inc. 2004-04-20 |
 MCARE 76082983 not registered Dead/Abandoned |
Davinci Technologies Inc. 2000-07-06 |
 MCARE 76082982 not registered Dead/Abandoned |
Davinci Technologies Inc. 2000-07-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.