MyDay
Lenses, Soft Contact, Daily Wear
CooperVision, Inc.
The following data is part of a premarket notification filed by Coopervision, Inc. with the FDA for Myday.
Pre-market Notification Details
| Device ID | K190965 |
| 510k Number | K190965 |
| Device Name: | MyDay |
| Classification | Lenses, Soft Contact, Daily Wear |
| Applicant | CooperVision, Inc. 5870 Stoneridge Drive, Suite 1 Pleasanton, CA 94588 |
| Contact | Marie Dutton |
| Correspondent | Marie Dutton CooperVision, Inc. 5870 Stoneridge Drive, Suite 1 Pleasanton, CA 94588 |
| Product Code | LPL |
| CFR Regulation Number | 886.5925 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Special |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2019-04-12 |
| Decision Date | 2019-04-29 |
Trademark Results [MyDay]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MYDAY 97606984 not registered Live/Pending |
Plexxis Software Inc. 2022-09-26 |
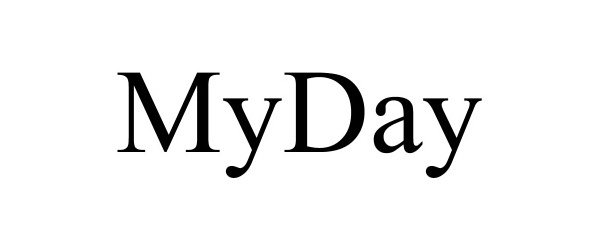 MYDAY 90023209 not registered Live/Pending |
Linkedrive, Inc. 2020-06-26 |
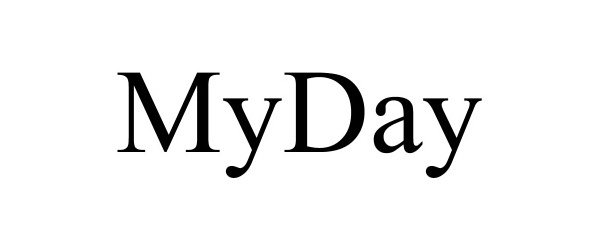 MYDAY 90023209 not registered Live/Pending |
PELMEA, LLP 2020-06-26 |
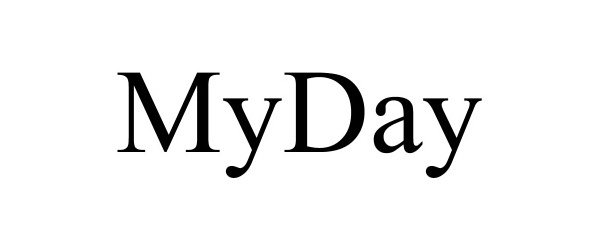 MYDAY 90023209 not registered Live/Pending |
TRUST FBO MARY EMMA CONRADES BAER UNDERTHE GARDENS/SPRUCE HEAD TRUST 2020-06-26 |
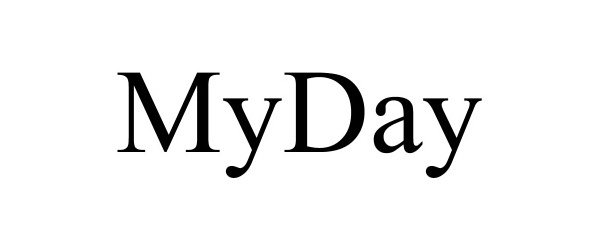 MYDAY 90023209 not registered Live/Pending |
MARY EMMA CONRADES BAER TRUST UNDER THEMAINSTREET TRUST 2020-06-26 |
 MYDAY 88524806 not registered Live/Pending |
AMICUS THERAPEUTICS, INC. 2019-07-19 |
 MYDAY 87301611 5421264 Live/Registered |
Collabco Ltd 2017-01-13 |
 MYDAY 87248332 5487453 Live/Registered |
SIOTOR, Monika 2016-11-27 |
 MYDAY 85907781 4813300 Live/Registered |
CooperVision, Inc. 2013-04-18 |
 MYDAY 85884390 not registered Dead/Abandoned |
Myday.org LLC 2013-03-22 |
 MYDAY 78261430 2925936 Dead/Cancelled |
Members Service Group, Inc. 2003-06-12 |
 MYDAY 78261427 2925935 Dead/Cancelled |
Members Service Group, Inc. 2003-06-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.