EZ-Cover
Transducer, Ultrasonic, Diagnostic
MedXPress.Pro
The following data is part of a premarket notification filed by Medxpress.pro with the FDA for Ez-cover.
Pre-market Notification Details
| Device ID | K191491 |
| 510k Number | K191491 |
| Device Name: | EZ-Cover |
| Classification | Transducer, Ultrasonic, Diagnostic |
| Applicant | MedXPress.Pro Slachthuislaan 1, Bus 102 Leuven, BE 3000 |
| Contact | Katherine Hughey |
| Correspondent | Jeff Kasoff Lean To Quality, LLC 107 Avenue A Willis, TX 77378 |
| Product Code | ITX |
| CFR Regulation Number | 892.1570 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2019-06-05 |
| Decision Date | 2020-06-05 |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 05407006211181 | K191491 | 000 |
| 05407006211631 | K191491 | 000 |
| 05407006211600 | K191491 | 000 |
| 05407006211570 | K191491 | 000 |
| 05407006211549 | K191491 | 000 |
| 05407006211518 | K191491 | 000 |
| 05407006211488 | K191491 | 000 |
| 05407006211457 | K191491 | 000 |
| 05407006211426 | K191491 | 000 |
| 05407006211396 | K191491 | 000 |
| 05407006211365 | K191491 | 000 |
| 05407006211334 | K191491 | 000 |
| 05407006211303 | K191491 | 000 |
| 05407006211273 | K191491 | 000 |
| 05407006211242 | K191491 | 000 |
| 05407006211662 | K191491 | 000 |
| 05407006210733 | K191491 | 000 |
| 05407006211150 | K191491 | 000 |
| 05407006211129 | K191491 | 000 |
| 05407006211099 | K191491 | 000 |
| 05407006211068 | K191491 | 000 |
| 05407006211037 | K191491 | 000 |
| 05407006211006 | K191491 | 000 |
| 05407006210979 | K191491 | 000 |
| 05407006210948 | K191491 | 000 |
| 05407006210917 | K191491 | 000 |
| 05407006210887 | K191491 | 000 |
| 05407006210856 | K191491 | 000 |
| 05407006210825 | K191491 | 000 |
| 05407006210795 | K191491 | 000 |
| 05407006210764 | K191491 | 000 |
| 05407006211211 | K191491 | 000 |
Trademark Results [EZ-Cover]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
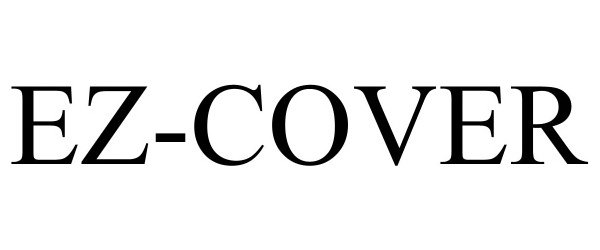 EZ-COVER 98010519 not registered Live/Pending |
Ningbo Weiang Information Technology Co., Ltd. 2023-05-24 |
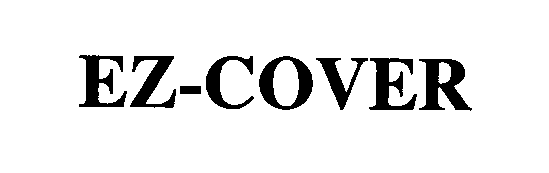 EZ-COVER 76433869 2713418 Live/Registered |
GERBER CHILDRENSWEAR LLC 2002-07-24 |
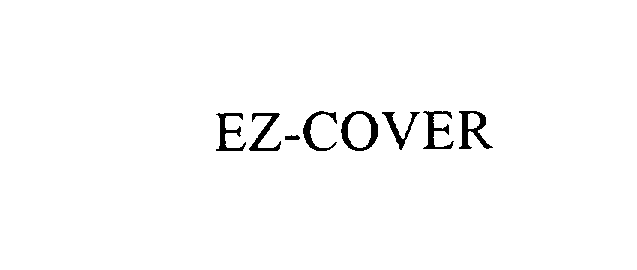 EZ-COVER 76028656 not registered Dead/Abandoned |
EZ-COVER Inc. 2000-04-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.