ProSeal™ Closed System Drug Transfer Device (CSTD)
Closed Antineoplastic And Hazardous Drug Reconstitution And Transfer System
Epic Medical Pte. Ltd.
The following data is part of a premarket notification filed by Epic Medical Pte. Ltd. with the FDA for Proseal™ Closed System Drug Transfer Device (cstd).
Pre-market Notification Details
| Device ID | K192075 |
| 510k Number | K192075 |
| Device Name: | ProSeal™ Closed System Drug Transfer Device (CSTD) |
| Classification | Closed Antineoplastic And Hazardous Drug Reconstitution And Transfer System |
| Applicant | Epic Medical Pte. Ltd. 10 Jalan Besar, #06-04, Sim Lim Tower Singapore, SG 208787 |
| Contact | Freddie Lee |
| Correspondent | Roshana Ahmed G&L Scientific, Inc. 25 Independence Blvd. Warren, NJ 07059 |
| Product Code | ONB |
| CFR Regulation Number | 880.5440 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2019-08-02 |
| Decision Date | 2020-08-07 |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 38859299124125 | K192075 | 000 |
Trademark Results [ProSeal]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
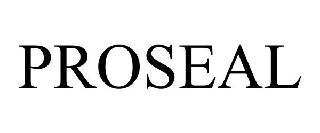 PROSEAL 98574834 not registered Live/Pending |
Thermofluid Technologies, Inc. 2024-05-29 |
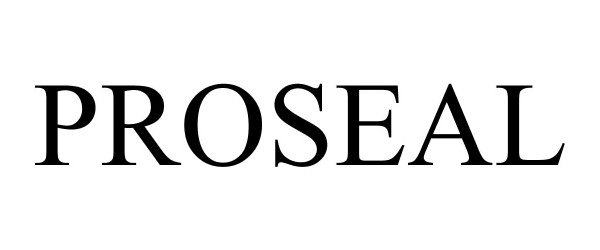 PROSEAL 86689537 5115384 Live/Registered |
TELEFLEX LIFE SCIENCES UNLIMITED COMPANY 2015-07-10 |
 PROSEAL 85123375 not registered Dead/Abandoned |
Auto Care Products, Inc. 2010-09-04 |
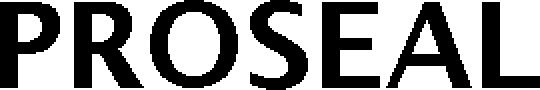 PROSEAL 79191154 5308741 Live/Registered |
Proseal UK Ltd 2016-07-05 |
 PROSEAL 79090895 4008236 Live/Registered |
Otto Bock HealthCare GmbH 2010-10-22 |
 PROSEAL 78850068 not registered Dead/Abandoned |
Verimark (Pty) Limited 2006-03-30 |
 PROSEAL 78391278 not registered Dead/Abandoned |
Provencher Plumbing and Heating 2004-03-26 |
 PROSEAL 78300757 not registered Dead/Abandoned |
ProSeal Chicago, Inc. 2003-09-16 |
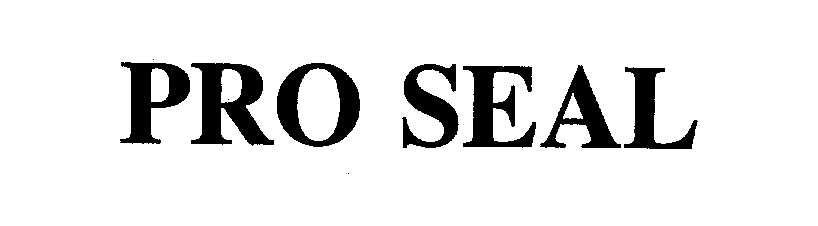 PROSEAL 78165113 2953070 Live/Registered |
DELAWARE CAPITAL FORMATION, INC. 2002-09-17 |
 PROSEAL 76542026 not registered Dead/Abandoned |
BRIDGESTONE CORPORATION 2003-09-02 |
 PROSEAL 76428088 not registered Dead/Abandoned |
TRIM-GARD COMPANY LIMITED 2002-07-08 |
 PROSEAL 76337950 not registered Dead/Abandoned |
BRIDGESTONE CORPORATION 2001-11-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.