I Do
Implant, Endosseous, Root-form
I Do Biotech Co., Ltd
The following data is part of a premarket notification filed by I Do Biotech Co., Ltd with the FDA for I Do.
Pre-market Notification Details
| Device ID | K192294 |
| 510k Number | K192294 |
| Device Name: | I Do |
| Classification | Implant, Endosseous, Root-form |
| Applicant | I Do Biotech Co., Ltd #C, 135, Seongseodong-ro, Dalsoe-gu Daegu, KR 42721 |
| Contact | Jae-hyung Song |
| Correspondent | April Lee Withus Group Inc 106 Superior Irvine, CA 92620 |
| Product Code | DZE |
| CFR Regulation Number | 872.3640 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2019-08-23 |
| Decision Date | 2020-07-31 |
Trademark Results [I Do]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 I DO 98891096 not registered Live/Pending |
Huizhou Youxia Technology Co., Ltd. 2024-12-08 |
 I DO 90265028 not registered Live/Pending |
WinLove Trading Co., Ltd. 2020-10-20 |
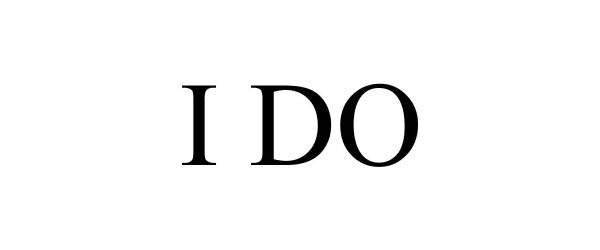 I DO 88125593 not registered Live/Pending |
Fish & Wildlife Foundation of Florida, Inc. 2018-09-20 |
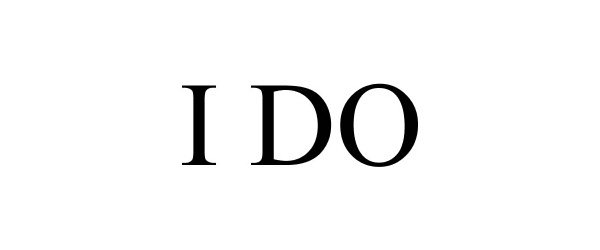 I DO 88116182 not registered Live/Pending |
Heather Cohen and Eva Mongie, a partnership 2018-09-13 |
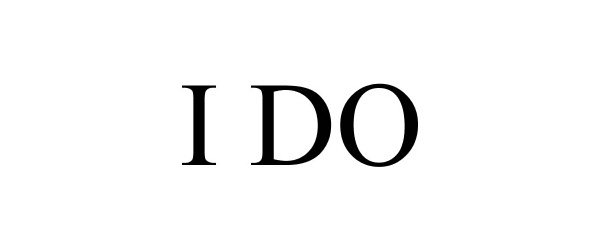 I DO 87548520 not registered Dead/Abandoned |
Anat Safir 2017-07-30 |
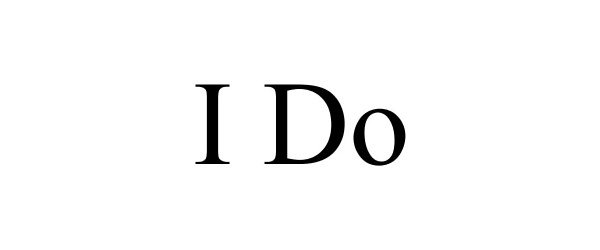 I DO 87140450 5242925 Live/Registered |
Albers Designs LLC 2016-08-16 |
 I DO 86450168 4764350 Live/Registered |
ZHUHAI IDO FURNITURE CO., LTD. 2014-11-10 |
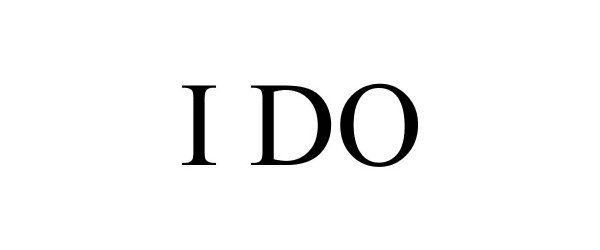 I DO 86409086 4890279 Live/Registered |
Paul A Fugazzotto, DDS, PC 2014-09-29 |
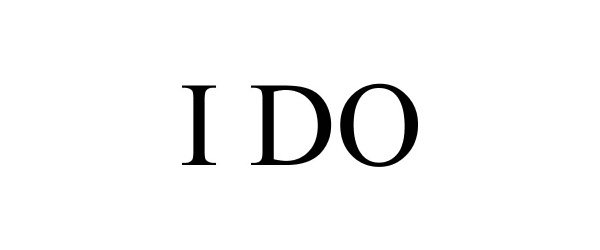 I DO 86050957 4472853 Live/Registered |
Phyxius Sports Inc. 2013-08-28 |
 I DO 86041176 not registered Live/Pending |
Medlars LLC 2013-08-19 |
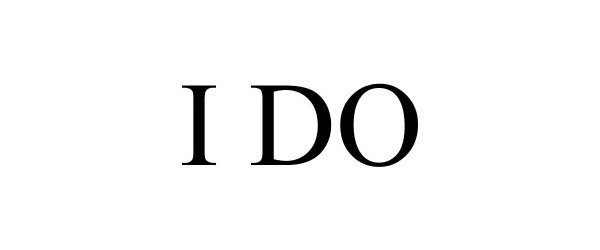 I DO 85572895 4221977 Dead/Cancelled |
Lifetime Brands, Inc. 2012-03-19 |
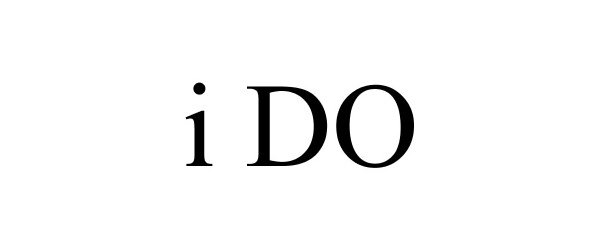 I DO 85337834 not registered Dead/Abandoned |
Smith, Jared J. 2011-06-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.