Spinalytics
System, Image Processing, Radiological
Optima Health Solutions International Corp.
The following data is part of a premarket notification filed by Optima Health Solutions International Corp. with the FDA for Spinalytics.
Pre-market Notification Details
| Device ID | K192629 |
| 510k Number | K192629 |
| Device Name: | Spinalytics |
| Classification | System, Image Processing, Radiological |
| Applicant | Optima Health Solutions International Corp. Unit 303 - 828 W 8th Avenue Vancouver, CA V5z 1e2 |
| Contact | Farhad Ghani |
| Correspondent | Diane Sudduth Emergo Global Consulting, LLC 2500 Bee Cave Road, Bldg. 1, Suite 300 Austin, TX 78746 |
| Product Code | LLZ |
| CFR Regulation Number | 892.2050 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2019-09-23 |
| Decision Date | 2019-12-21 |
Trademark Results [Spinalytics]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
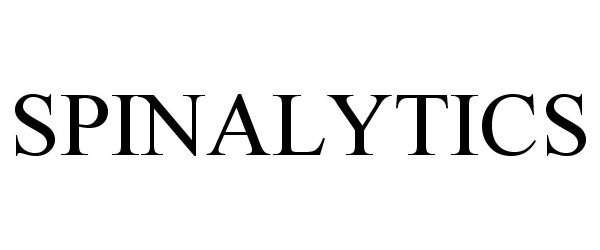 SPINALYTICS 86200180 not registered Dead/Abandoned |
Wave Treatment Holdings Pte. Ltd. 2014-02-21 |
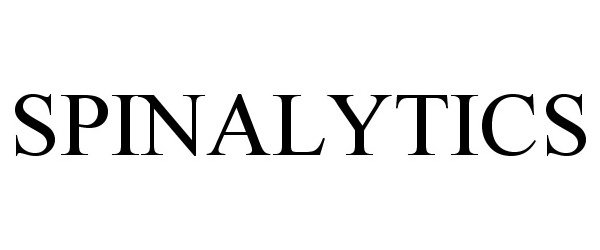 SPINALYTICS 85471252 4157903 Dead/Cancelled |
Optima Health Solutions International Corporation 2011-11-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.