V-Mix
Cleanser, Root Canal
Inter-Med/ Vista Dental Products
The following data is part of a premarket notification filed by Inter-med/ Vista Dental Products with the FDA for V-mix.
Pre-market Notification Details
| Device ID | K193357 |
| 510k Number | K193357 |
| Device Name: | V-Mix |
| Classification | Cleanser, Root Canal |
| Applicant | Inter-Med/ Vista Dental Products 2220 South Street Suite A Racine, WI 53404 |
| Contact | Katherine Barry |
| Correspondent | Katherine Barry Inter-Med/ Vista Dental Products 2220 South Street Suite A Racine, WI 53404 |
| Product Code | KJJ |
| CFR Regulation Number | 510(k) Premarket Notification [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2019-12-04 |
| Decision Date | 2020-10-23 |
Trademark Results [V-Mix]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
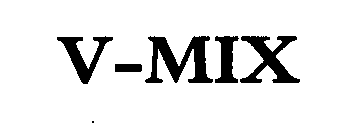 V-MIX 76340131 2835816 Dead/Cancelled |
Accentus plc 2001-11-20 |
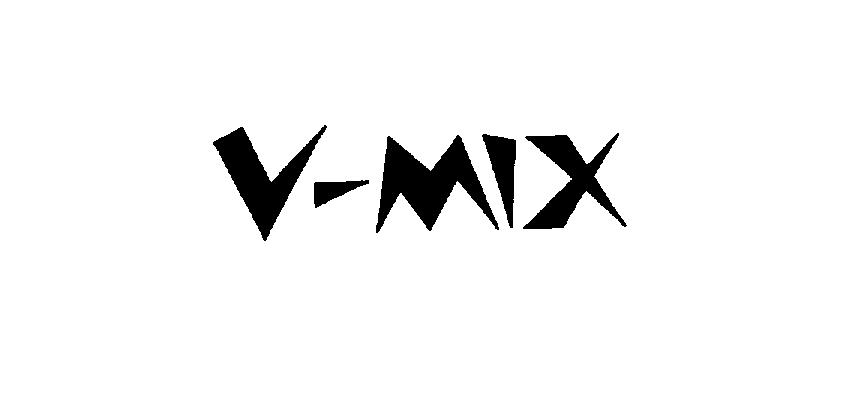 V-MIX 75896971 2878128 Dead/Cancelled |
HOLIDAY ENTERTAINMENT CO., LTD. 2000-01-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.