PRE-SURE
System, Image Processing, Radiological
Lazarus, 3D, Inc
The following data is part of a premarket notification filed by Lazarus, 3d, Inc with the FDA for Pre-sure.
Pre-market Notification Details
| Device ID | K201835 |
| 510k Number | K201835 |
| Device Name: | PRE-SURE |
| Classification | System, Image Processing, Radiological |
| Applicant | Lazarus, 3D, Inc 3513 NW Mink Pl Corvallis, OR 97330 |
| Contact | Smriti Zanevald |
| Correspondent | Elisa Maldonado-holmertz Obelix Consulting 12416 Fairfax Ridge Place Austin, TX 78738 |
| Product Code | LLZ |
| CFR Regulation Number | 892.2050 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2020-07-02 |
| Decision Date | 2021-07-08 |
Trademark Results [PRE-SURE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PRE-SURE 98212393 not registered Live/Pending |
Central States Industrial Equipment & Service, Inc. 2023-10-06 |
 PRE-SURE 98169457 not registered Live/Pending |
Lake Products Limited 2023-09-07 |
 PRE-SURE 90307808 not registered Live/Pending |
LAZARUS 3D 2020-11-09 |
 PRE-SURE 79400926 not registered Live/Pending |
LAKE PRODUCTS LIMITED 2024-03-04 |
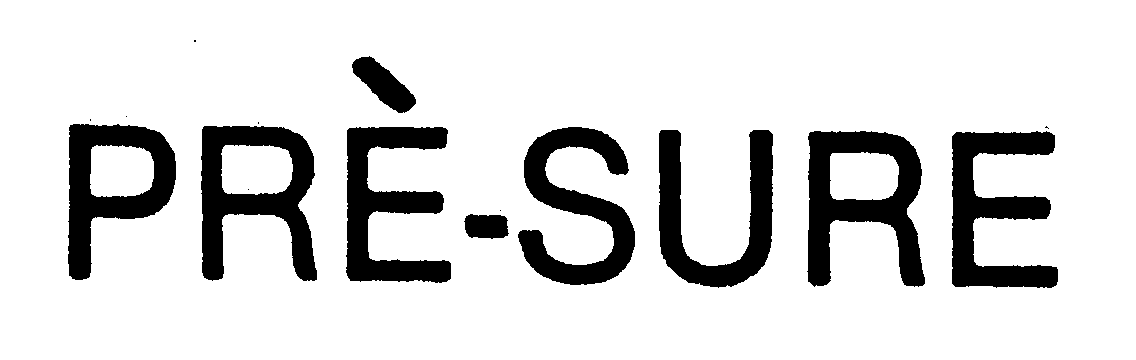 PRE-SURE 74257390 1756640 Dead/Cancelled |
Accu-Dent 1992-03-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.