B-Capta
Monitor, Blood-gas, On-line, Cardiopulmonary Bypass
LivaNova Deutschland GmbH
The following data is part of a premarket notification filed by Livanova Deutschland Gmbh with the FDA for B-capta.
Pre-market Notification Details
| Device ID | K202154 |
| 510k Number | K202154 |
| Device Name: | B-Capta |
| Classification | Monitor, Blood-gas, On-line, Cardiopulmonary Bypass |
| Applicant | LivaNova Deutschland GmbH Lindberghstr. 25 Munich, DE 80939 |
| Contact | Mattia Ronchetti |
| Correspondent | Mattia Ronchetti LivaNova Deutschland GmbH Lindberghstr. 25 Munich, DE 80939 |
| Product Code | DRY |
| CFR Regulation Number | 870.4330 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2020-08-03 |
| Decision Date | 2021-04-01 |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 38033178113527 | K202154 | 000 |
| 04033817901624 | K202154 | 000 |
| 04033817901631 | K202154 | 000 |
| 04033817902836 | K202154 | 000 |
| 04033817903000 | K202154 | 000 |
| 38033178113091 | K202154 | 000 |
| 38033178113107 | K202154 | 000 |
| 38033178113114 | K202154 | 000 |
| 38033178113510 | K202154 | 000 |
| 04033817901617 | K202154 | 000 |
Trademark Results [B-Capta]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
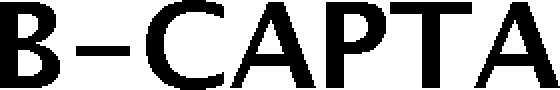 B-CAPTA 79240105 5724415 Live/Registered |
SORIN GROUP ITALIA S.R.L. 2018-06-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.