Artus™ Cervical Plate System
Appliance, Fixation, Spinal Intervertebral Body
Ulrich GmbH & Co. KG
The following data is part of a premarket notification filed by Ulrich Gmbh & Co. Kg with the FDA for Artus™ Cervical Plate System.
Pre-market Notification Details
| Device ID | K202227 |
| 510k Number | K202227 |
| Device Name: | Artus™ Cervical Plate System |
| Classification | Appliance, Fixation, Spinal Intervertebral Body |
| Applicant | Ulrich GmbH & Co. KG Buchbrunnenweg 12 Ulm, DE 89081 |
| Contact | Christoph Ulrich |
| Correspondent | Hans Stover Ulrich Medical USA 18221 Edison Avenue Chesterfield, MO 63005 |
| Product Code | KWQ |
| CFR Regulation Number | 888.3060 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2020-08-07 |
| Decision Date | 2020-09-14 |
Trademark Results [Artus]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ARTUS 90649863 not registered Live/Pending |
Radyne Corporation 2021-04-16 |
 ARTUS 79341014 not registered Live/Pending |
MyoPowers Medical Technologies France 2022-04-21 |
 ARTUS 79126953 4449572 Live/Registered |
MyoPowers Medical Technologies France 2013-01-24 |
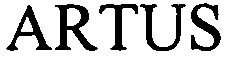 ARTUS 79060103 3689584 Live/Registered |
QIAGEN Hamburg GmbH 2008-06-25 |
 ARTUS 76350403 3022357 Dead/Cancelled |
QIAGEN HAMBURG GMBH 2001-12-20 |
 ARTUS 76163939 2847887 Dead/Cancelled |
DESTYLARNIA SOBIESKI S.A. 2000-11-06 |
 ARTUS 75256744 2346293 Dead/Cancelled |
Nokia Telecommunications Oy 1997-03-13 |
 ARTUS 74633351 1945806 Dead/Cancelled |
ACUFEX MICROSURGICAL, INC. 1995-02-13 |
 ARTUS 74237142 not registered Dead/Abandoned |
UNIX SYSTEM LABORATORIES, INC. 1992-01-13 |
 ARTUS 73659056 1498492 Dead/Cancelled |
ACUFEX MICROSURGICAL, INC. 1987-05-05 |
 ARTUS 71499497 0439512 Live/Registered |
INDUSTRIAL PRODUCTS SUPPLIERS 1946-04-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.