The Slide
Device, Jaw Repositioning
Biotex, Inc.
The following data is part of a premarket notification filed by Biotex, Inc. with the FDA for The Slide.
Pre-market Notification Details
| Device ID | K203712 |
| 510k Number | K203712 |
| Device Name: | The Slide |
| Classification | Device, Jaw Repositioning |
| Applicant | Biotex, Inc. 114 Holmes Rd. Houston, TX 77045 |
| Contact | Wade Munsch |
| Correspondent | Wade Munsch Biotex, Inc. 114 Holmes Rd. Houston, TX 77045 |
| Product Code | LQZ |
| CFR Regulation Number | 872.5570 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2020-12-21 |
| Decision Date | 2021-07-20 |
| Summary: | summary |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| M71004610000 | K203712 | 000 |
| M71004615000 | K203712 | 000 |
Trademark Results [The Slide]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
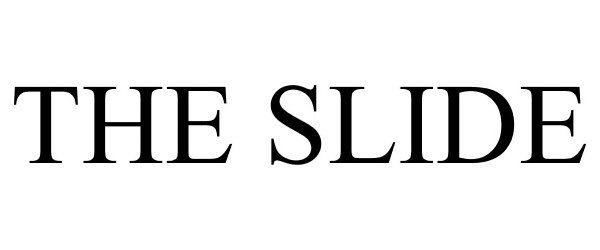 THE SLIDE 87868243 not registered Live/Pending |
LeBlanc Dental Products, Inc. 2018-04-09 |
 THE SLIDE 78280158 2921725 Dead/Cancelled |
Mancinelli, Ricci 2003-07-29 |
 THE SLIDE 78128460 not registered Dead/Abandoned |
international bullion and metal brokers(usa) inc 2002-05-14 |
 THE SLIDE 74371263 not registered Dead/Abandoned |
Fitness Innovations, Inc. 1993-03-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.