Dayspring
Sleeve, Limb, Compressible
Koya Medical, Inc.
The following data is part of a premarket notification filed by Koya Medical, Inc. with the FDA for Dayspring.
Pre-market Notification Details
| Device ID | K210885 |
| 510k Number | K210885 |
| Device Name: | Dayspring |
| Classification | Sleeve, Limb, Compressible |
| Applicant | Koya Medical, Inc. 2461 Peralta St. Oakland, CA 94607 |
| Contact | Andy Doraiswamy |
| Correspondent | Alex Chang BioDesign Regulatory Services, LLC 16185 Los Gatos Blvd. Los Gatos, CA 95032 |
| Product Code | JOW |
| CFR Regulation Number | 870.5800 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Special |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2021-03-25 |
| Decision Date | 2021-04-23 |
Trademark Results [Dayspring]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
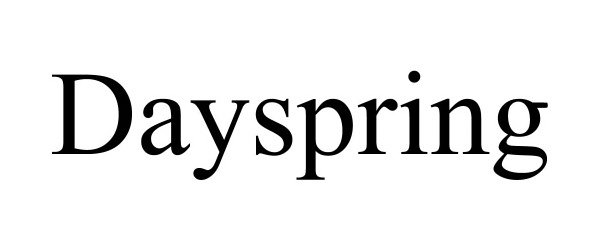 DAYSPRING 90152250 not registered Live/Pending |
FGBC, LLC 2020-09-01 |
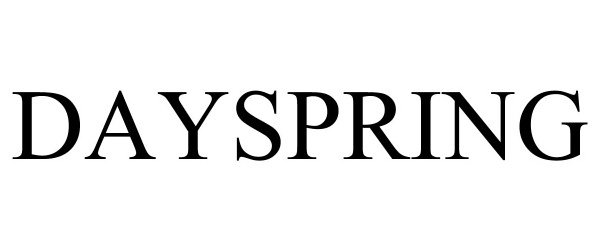 DAYSPRING 88730687 not registered Live/Pending |
KOYA, INC. 2019-12-17 |
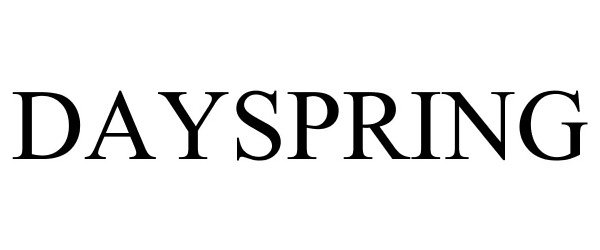 DAYSPRING 87701472 5852321 Live/Registered |
DaySpring Cards, Inc. 2017-11-29 |
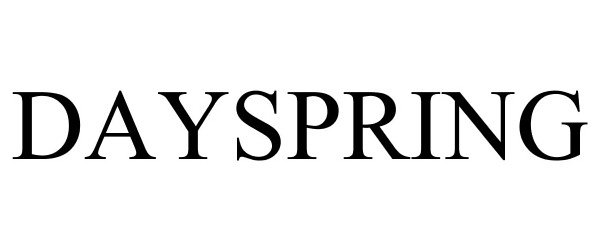 DAYSPRING 87603735 5602798 Live/Registered |
DaySpring Cards, Inc. 2017-09-11 |
 DAYSPRING 78766890 not registered Dead/Abandoned |
Dayspring and Associates 2005-12-05 |
 DAYSPRING 78708181 3112124 Live/Registered |
DaySpring Cards, Inc. 2005-09-07 |
 DAYSPRING 78708157 3112123 Live/Registered |
DaySpring Cards, Inc. 2005-09-07 |
 DAYSPRING 78687844 3112119 Live/Registered |
DaySpring Cards, Inc. 2005-08-08 |
 DAYSPRING 78679817 3112096 Live/Registered |
DaySpring Cards, Inc. 2005-07-27 |
 DAYSPRING 78679791 3112094 Live/Registered |
DaySpring Cards, Inc. 2005-07-27 |
 DAYSPRING 78679766 3112093 Live/Registered |
DaySpring Cards, Inc. 2005-07-27 |
 DAYSPRING 78679727 3112090 Live/Registered |
DaySpring Cards, Inc. 2005-07-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.