PlasmaWave
Sleeve, Limb, Compressible
ManaMed, Inc.
The following data is part of a premarket notification filed by Manamed, Inc. with the FDA for Plasmawave.
Pre-market Notification Details
| Device ID | K211253 |
| 510k Number | K211253 |
| Device Name: | PlasmaWave |
| Classification | Sleeve, Limb, Compressible |
| Applicant | ManaMed, Inc. 5240 W. Charleston Blvd. Las Vegas, NV 89146 |
| Contact | Trevor Theriot |
| Correspondent | Bill Quanqin Dai JKH USA, LLC 14271 Jeffrey Rd. #246 Irvine, CA 92620 |
| Product Code | JOW |
| CFR Regulation Number | 870.5800 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Special |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2021-04-26 |
| Decision Date | 2021-05-26 |
Trademark Results [PlasmaWave]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
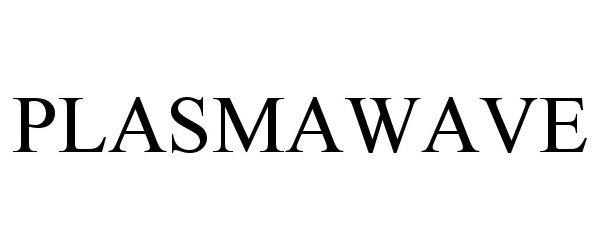 PLASMAWAVE 90802973 not registered Live/Pending |
MANAMED INC 2021-06-30 |
 PLASMAWAVE 90101057 not registered Live/Pending |
WINIX INC. 2020-08-07 |
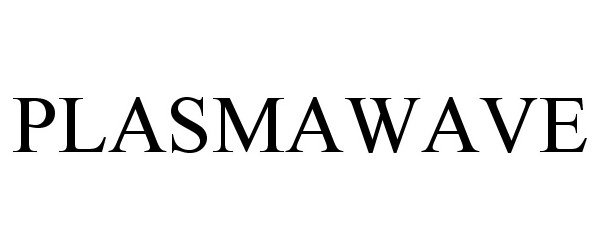 PLASMAWAVE 77549769 3685151 Live/Registered |
WINIX Inc. 2008-08-18 |
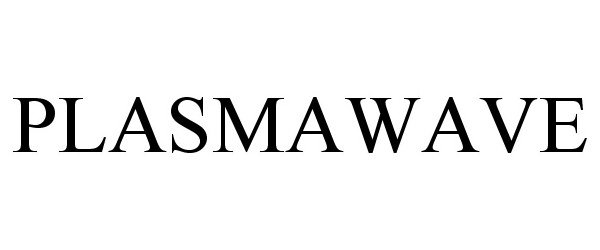 PLASMAWAVE 77548689 not registered Dead/Abandoned |
WINIX Inc. 2008-08-15 |
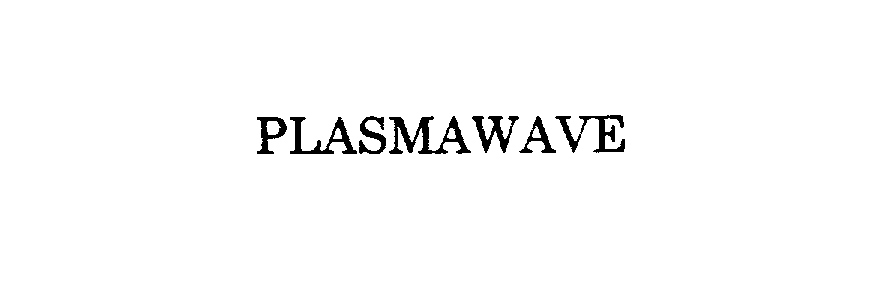 PLASMAWAVE 76092080 not registered Dead/Abandoned |
Fiberstars, Inc. 2000-07-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.