S-Line
Warmer, Thermal, Infusion Fluid
Barkey GmbH & Co. KG
The following data is part of a premarket notification filed by Barkey Gmbh & Co. Kg with the FDA for S-line.
Pre-market Notification Details
| Device ID | K213191 |
| 510k Number | K213191 |
| Device Name: | S-Line |
| Classification | Warmer, Thermal, Infusion Fluid |
| Applicant | Barkey GmbH & Co. KG Gewerbestr. 8 Leopoldshoehe, DE 33818 |
| Contact | Thomas Barkey |
| Correspondent | Paul Dryden ProMedic Consulting LLC 131 Bay Point Dr NE Saint Petersburg, FL 33704 |
| Product Code | LGZ |
| CFR Regulation Number | 880.5725 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2021-09-29 |
| Decision Date | 2022-05-24 |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 04059201002203 | K213191 | 000 |
Trademark Results [S-Line]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 S-LINE 88115910 5886727 Live/Registered |
Nikon Corporation 2018-09-13 |
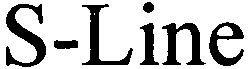 S-LINE 79121202 4441823 Live/Registered |
Bircher Reglomat AG 2012-08-31 |
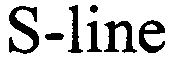 S-LINE 79089625 4011055 Live/Registered |
Volker Barkey 2010-07-14 |
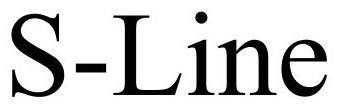 S-LINE 77393870 3636238 Dead/Cancelled |
Integrated Illumination Systems Inc. 2008-02-11 |
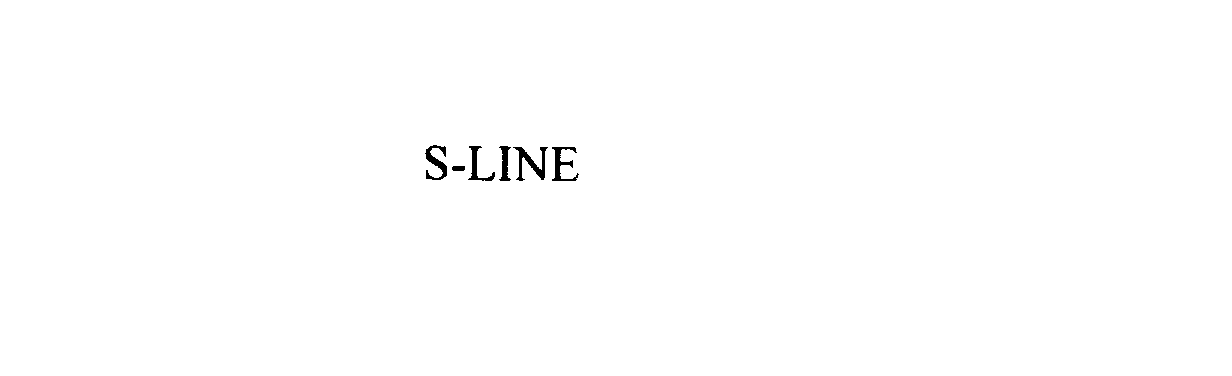 S-LINE 76081919 2651127 Live/Registered |
OBCORP LLC 2000-06-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.