Apyx
Fixation, Non-absorbable Or Absorbable, For Pelvic Use
Escala Medical
The following data is part of a premarket notification filed by Escala Medical with the FDA for Apyx.
Pre-market Notification Details
| Device ID | K213783 |
| 510k Number | K213783 |
| Device Name: | Apyx |
| Classification | Fixation, Non-absorbable Or Absorbable, For Pelvic Use |
| Applicant | Escala Medical 17th Tchelet Street Misgav Industrial Park, IL 2017400 |
| Contact | Edit Goldberg |
| Correspondent | Jonathan Kahan Hogan Lovells 555 Thirteenth Street, NW Washington, DC 20004 -1109 |
| Product Code | PBQ |
| CFR Regulation Number | 884.4530 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2021-12-03 |
| Decision Date | 2022-04-05 |
NIH GUDID Devices
| Device Identifier | submissionNumber | Supplement |
|---|---|---|
| 07290019174066 | K213783 | 000 |
| 07290019174103 | K213783 | 000 |
| 07290019174110 | K213783 | 000 |
| 07290019174127 | K213783 | 000 |
| 07290019174134 | K213783 | 000 |
| 07290019174141 | K213783 | 000 |
| 07290019174158 | K213783 | 000 |
| 07290019174165 | K213783 | 000 |
| 07290019174172 | K213783 | 000 |
| 07290019174189 | K213783 | 000 |
| 07290019174196 | K213783 | 000 |
| 07290019174202 | K213783 | 000 |
| 07290019174035 | K213783 | 000 |
| 07290019174042 | K213783 | 000 |
| 07290019174059 | K213783 | 000 |
| 07290019174028 | K213783 | 000 |
| 07290019174011 | K213783 | 000 |
| 07290019174097 | K213783 | 000 |
Trademark Results [Apyx]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
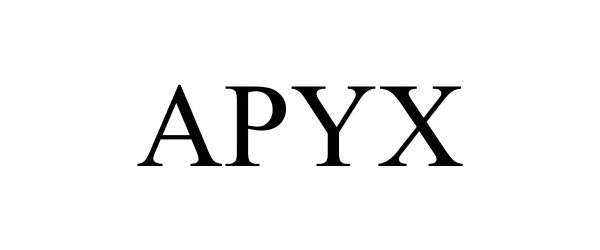 APYX 88225303 not registered Live/Pending |
Bovie Medical Corporation 2018-12-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.