CoLumbo
Automated Radiological Image Processing Software
Smart Soft Healthcare AD
The following data is part of a premarket notification filed by Smart Soft Healthcare Ad with the FDA for Columbo.
Pre-market Notification Details
| Device ID | K220497 |
| 510k Number | K220497 |
| Device Name: | CoLumbo |
| Classification | Automated Radiological Image Processing Software |
| Applicant | Smart Soft Healthcare AD 113 General Kolev Str. Primorski Distr., Office 7.2 Varna, BG 9002 |
| Contact | Yoana Ivanova |
| Correspondent | Yu Zhao LightSource Research LLC 2108 N St., Suite N Sacramento, CA 95816 |
| Product Code | QIH |
| CFR Regulation Number | 892.2050 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 2022-02-22 |
| Decision Date | 2022-06-23 |
Trademark Results [CoLumbo]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COLUMBO 85604494 4222022 Dead/Cancelled |
4 S Technologies, LLC 2012-04-20 |
 COLUMBO 85532262 4358809 Live/Registered |
Integrated Biometrics, LLC 2012-02-02 |
 COLUMBO 85273410 4124349 Live/Registered |
4 S Technologies, LLC 2011-03-22 |
 COLUMBO 78022346 not registered Dead/Abandoned |
Wisdom, Ali 2000-08-22 |
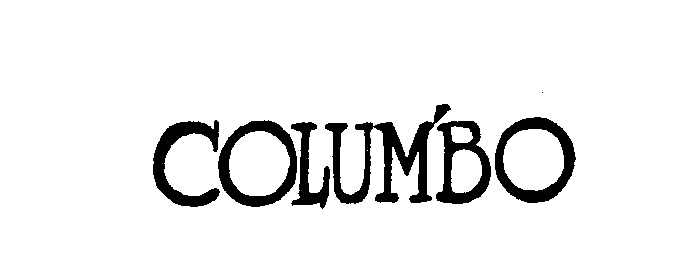 COLUMBO 73096773 1080568 Dead/Cancelled |
Rubyrose Incorporated 1977-08-16 |
 COLUMBO 73064494 1065454 Live/Registered |
UNIVERSAL CITY STUDIOS, INC. 1975-09-29 |
 COLUMBO 71233557 0220760 Dead/Expired |
1926-06-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.