PROCAST
Bandage, Cast
MEDICAL SPECIALTIES, INC.
The following data is part of a premarket notification filed by Medical Specialties, Inc. with the FDA for Procast.
Pre-market Notification Details
| Device ID | K791831 |
| 510k Number | K791831 |
| Device Name: | PROCAST |
| Classification | Bandage, Cast |
| Applicant | MEDICAL SPECIALTIES, INC. 803 N. Front St. Suite 3 Mchenry, IL 60050 |
| Product Code | ITG |
| CFR Regulation Number | 890.3025 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1979-09-18 |
| Decision Date | 1979-09-24 |
Trademark Results [PROCAST]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
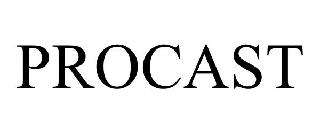 PROCAST 98592061 not registered Live/Pending |
Slide Insurance Holdings, Inc. 2024-06-09 |
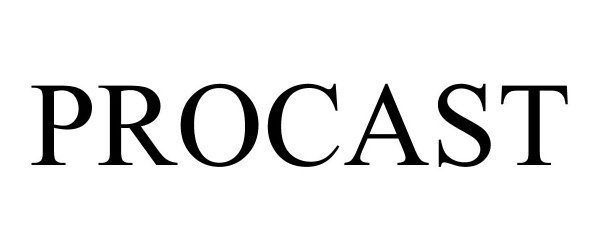 PROCAST 90979301 not registered Live/Pending |
American Bath Group, LLC 2021-02-04 |
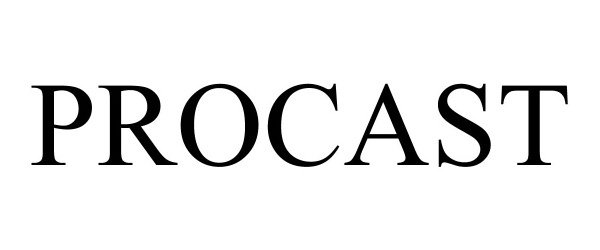 PROCAST 90512065 not registered Live/Pending |
American Bath Group, LLC 2021-02-04 |
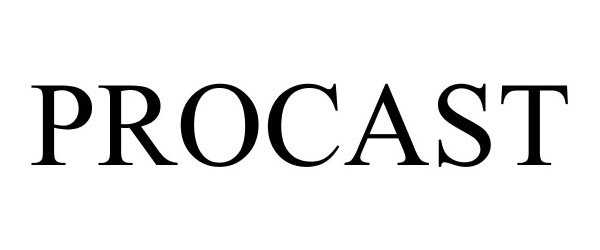 PROCAST 87484057 not registered Dead/Abandoned |
PMC Supplies, LLC 2017-06-12 |
 PROCAST 85911155 4465186 Live/Registered |
Miktek, LLC 2013-04-22 |
 PROCAST 85911152 4469427 Live/Registered |
Miktek, LLC 2013-04-22 |
 PROCAST 85691024 4385414 Live/Registered |
MEDITECH GROUP, LLC 2012-07-31 |
 PROCAST 79229211 5527178 Live/Registered |
ZHEJIANG PROCAST INDUSTRIAL CO., LTD 2017-12-14 |
 PROCAST 78351379 2923076 Live/Registered |
ITT MANUFACTURING ENTERPRISES, LLC 2004-01-13 |
 PROCAST 78265773 2844991 Dead/Cancelled |
CORSTONE, INC. 2003-06-23 |
 PROCAST 78141614 not registered Dead/Abandoned |
Brown, Marshall L. 2002-07-06 |
 PROCAST 78141614 not registered Dead/Abandoned |
Light, Theodore 2002-07-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.