TEMPO
Alloy, Other Noble Metal
DERINGER-NEY INC.
The following data is part of a premarket notification filed by Deringer-ney Inc. with the FDA for Tempo.
Pre-market Notification Details
| Device ID | K792274 |
| 510k Number | K792274 |
| Device Name: | TEMPO |
| Classification | Alloy, Other Noble Metal |
| Applicant | DERINGER-NEY INC. 803 N. Front St. Suite 3 Mchenry, IL 60050 |
| Product Code | EJS |
| CFR Regulation Number | 872.3060 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1979-11-13 |
| Decision Date | 1979-11-30 |
Trademark Results [TEMPO]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TEMPO 98752518 not registered Live/Pending |
Responsive Environments Inc. 2024-09-16 |
 TEMPO 98740633 not registered Live/Pending |
Eli Lilly and Company 2024-09-09 |
 TEMPO 98687902 not registered Live/Pending |
McBarnette Neilley, Cathy L 2024-08-07 |
 TEMPO 98467788 not registered Live/Pending |
TEMPO ORGAN PROCUREMENT PROFESSIONALS, LLC 2024-03-26 |
 TEMPO 98460502 not registered Live/Pending |
Pace Surgical, Inc. 2024-03-21 |
 TEMPO 98438084 not registered Live/Pending |
GHP Group, Inc. 2024-03-07 |
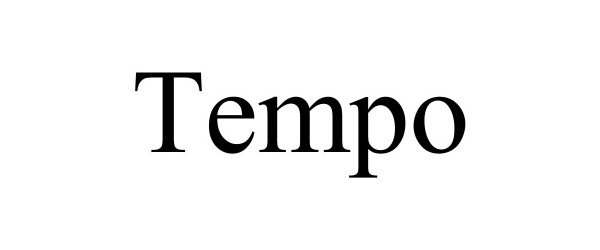 TEMPO 98346854 not registered Live/Pending |
Ergodriven Inc 2024-01-08 |
 TEMPO 98272495 not registered Live/Pending |
Capital One Financial Corporation 2023-11-15 |
 TEMPO 98258052 not registered Live/Pending |
AVY Entertainment, Inc. 2023-11-07 |
 TEMPO 98258051 not registered Live/Pending |
AVY Entertainment, Inc. 2023-11-07 |
 TEMPO 98258047 not registered Live/Pending |
AVY Entertainment, Inc. 2023-11-07 |
 TEMPO 98258043 not registered Live/Pending |
AVY Entertainment, Inc. 2023-11-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.