OPRAFLEX
Dressing, Wound, Drug
SELOMAS, INC.
The following data is part of a premarket notification filed by Selomas, Inc. with the FDA for Opraflex.
Pre-market Notification Details
| Device ID | K812501 |
| 510k Number | K812501 |
| Device Name: | OPRAFLEX |
| Classification | Dressing, Wound, Drug |
| Applicant | SELOMAS, INC. 4221 Richmond Rd., N.W. Walker, MI 49534 |
| Product Code | FRO |
| CFR Regulation Number | 510(k) Premarket Notification [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1981-08-31 |
| Decision Date | 1981-10-02 |
Trademark Results [OPRAFLEX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
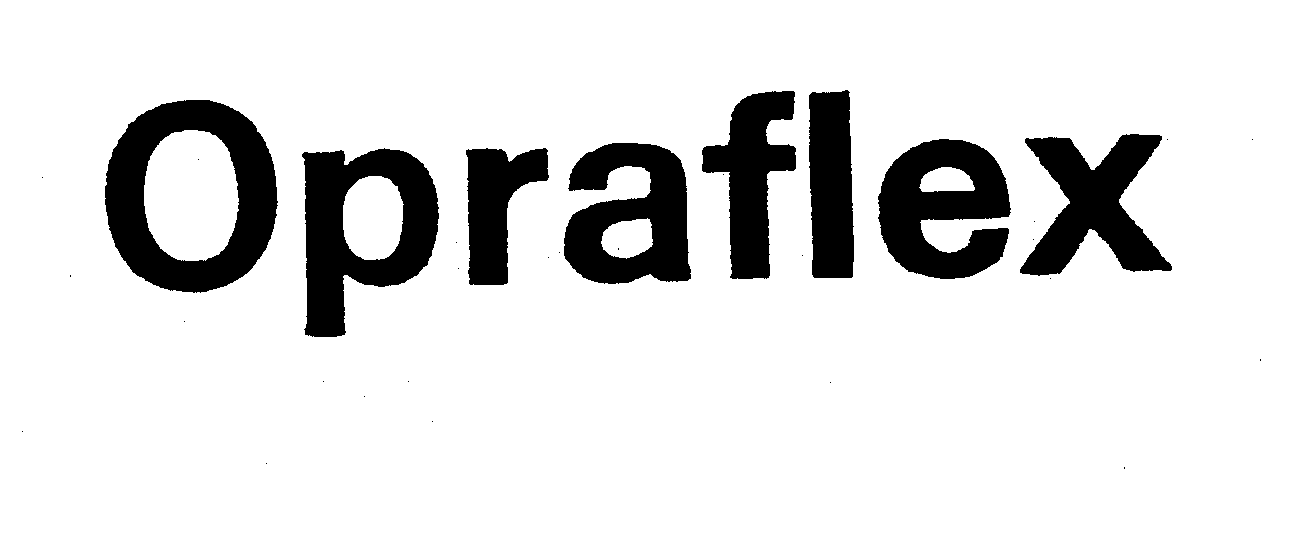 OPRAFLEX 73252578 1212309 Dead/Cancelled |
Lohmann GmbH & Co KG 1980-03-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.