CAVITRON
Cement, Dental
G-C INTL. CORP.
The following data is part of a premarket notification filed by G-c Intl. Corp. with the FDA for Cavitron.
Pre-market Notification Details
| Device ID | K812568 |
| 510k Number | K812568 |
| Device Name: | CAVITRON |
| Classification | Cement, Dental |
| Applicant | G-C INTL. CORP. 4221 Richmond Rd., N.W. Walker, MI 49534 |
| Product Code | EMA |
| CFR Regulation Number | 872.3275 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1981-09-09 |
| Decision Date | 1981-09-25 |
Trademark Results [CAVITRON]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CAVITRON 78690478 not registered Dead/Abandoned |
Harris, Jerome Michael 2005-08-11 |
 CAVITRON 78271035 3149400 Live/Registered |
ISP INVESTMENTS LLC 2003-07-07 |
 CAVITRON 78066602 2649452 Live/Registered |
DENTSPLY SIRONA INC. 2001-05-31 |
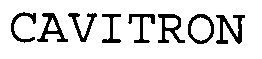 CAVITRON 76556241 not registered Dead/Abandoned |
HYDRO-THOMA LTD. 2003-10-23 |
 CAVITRON 74706899 1979835 Dead/Cancelled |
American Maize-Products Company 1995-07-27 |
 CAVITRON 73718673 1507977 Dead/Cancelled |
COOPER LASERSONICS, INC. 1988-03-25 |
 CAVITRON 73614732 1552725 Dead/Cancelled |
COOPERVISION, INC. 1986-08-14 |
 CAVITRON 71682396 0620009 Live/Registered |
CAVITRON CORPORATION 1955-02-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.