OPTI-MIST
Nebulizer (direct Patient Interface)
CORPAK CO.
The following data is part of a premarket notification filed by Corpak Co. with the FDA for Opti-mist.
Pre-market Notification Details
| Device ID | K820952 |
| 510k Number | K820952 |
| Device Name: | OPTI-MIST |
| Classification | Nebulizer (direct Patient Interface) |
| Applicant | CORPAK CO. 803 N. Front St. Suite 3 Mchenry, IL 60050 |
| Product Code | CAF |
| CFR Regulation Number | 868.5630 [🔎] |
| Decision | Substantially Equivalent (SESE) |
| Type | Traditional |
| 3rd Party Reviewed | No |
| Combination Product | No |
| Date Received | 1982-04-06 |
| Decision Date | 1982-04-29 |
Trademark Results [OPTI-MIST]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
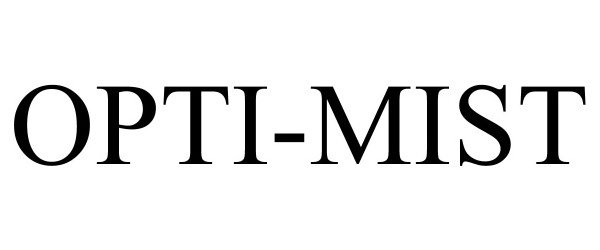 OPTI-MIST 97155787 not registered Live/Pending |
RefPlus Inc. 2021-12-03 |
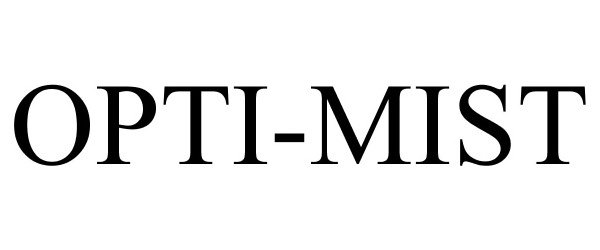 OPTI-MIST 85866735 not registered Dead/Abandoned |
Lancome Parfums et Beaute & Cie 2013-03-05 |
 OPTI-MIST 78127003 2794604 Dead/Cancelled |
Maersk Medical Inc. 2002-05-07 |
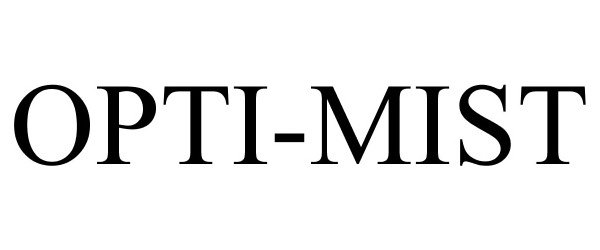 OPTI-MIST 77455306 3706216 Dead/Cancelled |
Duke Manufacturing Co. 2008-04-22 |
 OPTI-MIST 74680861 not registered Dead/Abandoned |
VALMET AUTOMATION (CANADA) LTD. 1995-05-30 |
 OPTI-MIST 74163971 not registered Dead/Abandoned |
VALMET AUTOMATION (CANADA) LTD. 1991-05-06 |
 OPTI-MIST 74117098 1666413 Dead/Cancelled |
BIOVEST, INC. 1990-11-20 |
 OPTI-MIST 73438436 1372330 Dead/Cancelled |
MEDIC-AID LIMITED 1983-08-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.